Magnesium Carbonate Content Analysis Service
- Store solid samples in a cool, dry, dark place; keep sealed.
- Store liquid samples in sealed cryogenic tubes; short‑term at 4 °C, long-term at –20 °C or below.
- Ship all samples under cold‑chain conditions (ice packs or dry ice) to prevent degradation or contamination.
Magnesium carbonate (MgCO₃) serves as a critical inorganic compound in both pharmaceutical formulation and basic lifescience research. As a buffering agent, antacid, and magnesium source, magnesium carbonate enhances drug bioavailability, stabilizes active ingredients, and supports therapeutic efficacy. In the field of nutrition, magnesium carbonate serves as a trusted mineral supplement that helps maintain healthy magnesium levels, promoting neuromuscular function and bone integrity. From drug delivery system development to the design of biocompatible materials, the purity and consistency of magnesium carbonate directly influence experimental validity and depth of scientific insight. Accurate quantification of magnesium carbonate content therefore represents a critical step in assuring both research reliability and product safety.
At MtoZ Biolabs, we understand that magnesium carbonate plays a pivotal role across pharmaceutical formulation, nutritional supplementation, and cuttingedge lifescience research. Our highprecision magnesium carbonate quantification service ensures that every batch of MgCO₃ meets the exacting standards required for drug development, biocompatible material design, and nutritional applications.
Our Analytical Platforms
ICP-OES
We leverage inductively coupled plasma optical emission spectroscopy to quantify magnesium in complex matrices with high sensitivity. This platform is ideal for routine testing of drug formulations, food ingredients, and biological samples, providing fast turnaround without compromising accuracy.
ICP-MS
For tracelevel quantitation, our inductively coupled plasma mass spectrometry workflow achieves detection limits at the partsperbillion level. This capability supports rigorous elemental profiling in biopharmaceutical development and demanding lifescience experiments.
XRF
Our Xray fluorescence spectrometers offer nondestructive screening of solid materials. When you need bulk screening of excipients or raw materials, XRF delivers rapid, costeffective confirmation of magnesium carbonate content without sample preparation.
TGA
Thermogravimetric analysis in our laboratory provides insight into the thermal stability and decomposition behavior of magnesium carbonate under controlled heating. These data support formulation stability studies and materialsscience research.
Why Choose MtoZ Biolabs?
At MtoZ Biolabs, our magnesium carbonate content analysis service is managed by a specialized technical team with deep expertise in inorganic analytics and pharmaceutical research. From method selection and sample preparation to data validation and interpretation, we deliver targeted recommendations that align with your research objectives.
✔ Comprehensive multiplatform support
Leveraging ICPOES, ICPMS, XRF, and TGA, we handle everything from raw MgCO₃ powders and finished formulations to complex biological matrices, ensuring the right technique for every sample type.
✔ Exceptional reproducibility
All workflows adhere to rigorous quality control standards, guaranteeing data that are reproducible, reliable, and ready for downstream application.
✔ Fast turnaround and flexible delivery
Standard project delivery occurs within five to seven business days. We also offer customized workflows and expedited options to keep your research milestones on track.
✔One-Time-Charge:
Our pricing is transparent, no hidden fees or additional costs.
✔ High-Data-Quality:
Deep data coverage with strict data quality control. AI-powered bioinformatics platform integrates all magnesium carbonate content analysis service data, providing clients with a comprehensive data report.
Analysis Workflow
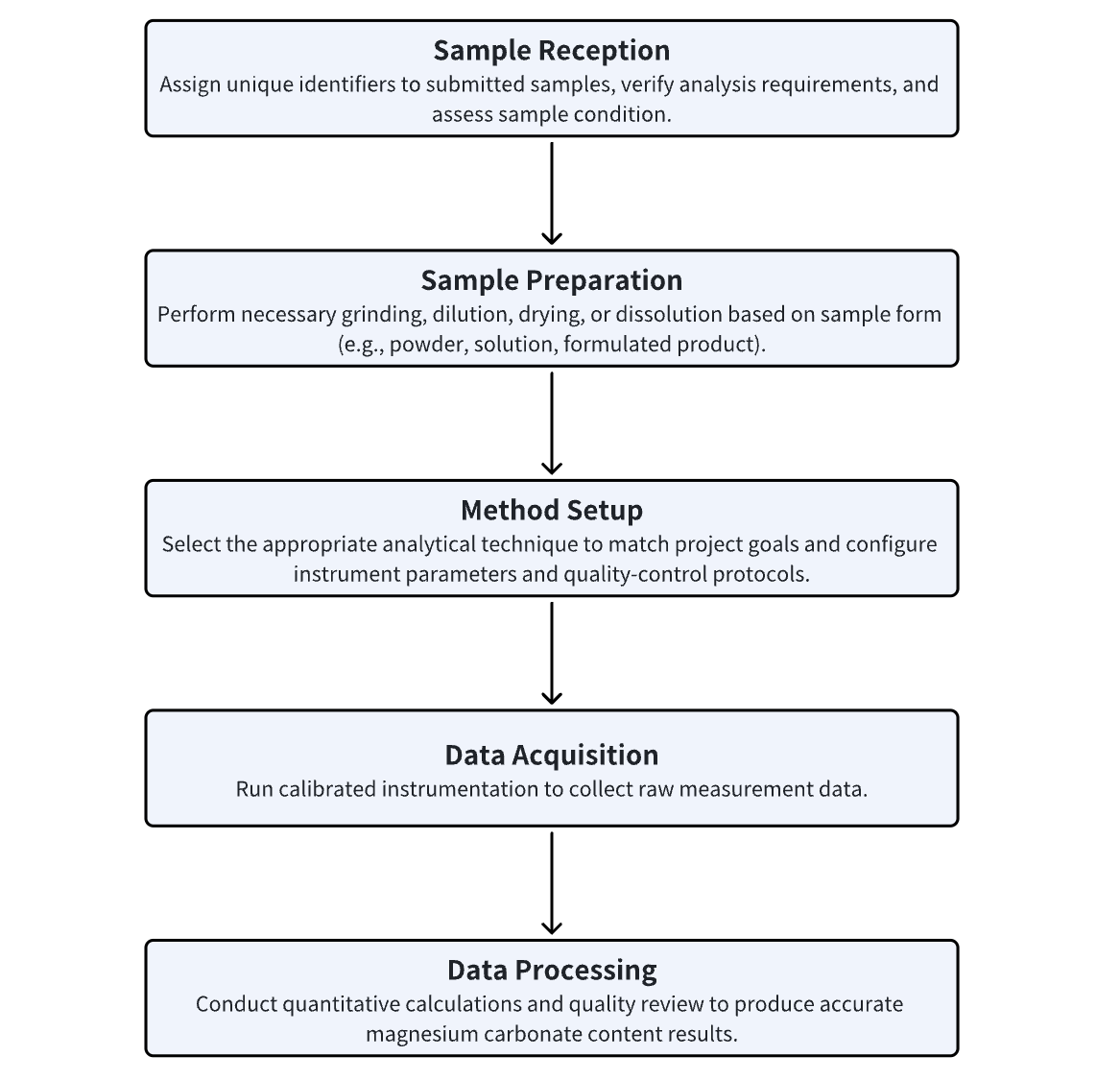
Applications
Quality control and consistency assessment of pharmaceutical excipients
Research and development of nutritional supplements and functional foods
Identification of active ingredients in raw materials and intermediates
Evaluation of biocompatible materials and formulation studies
Investigation of drug stability and degradation behavior
Sample Submission Suggestions
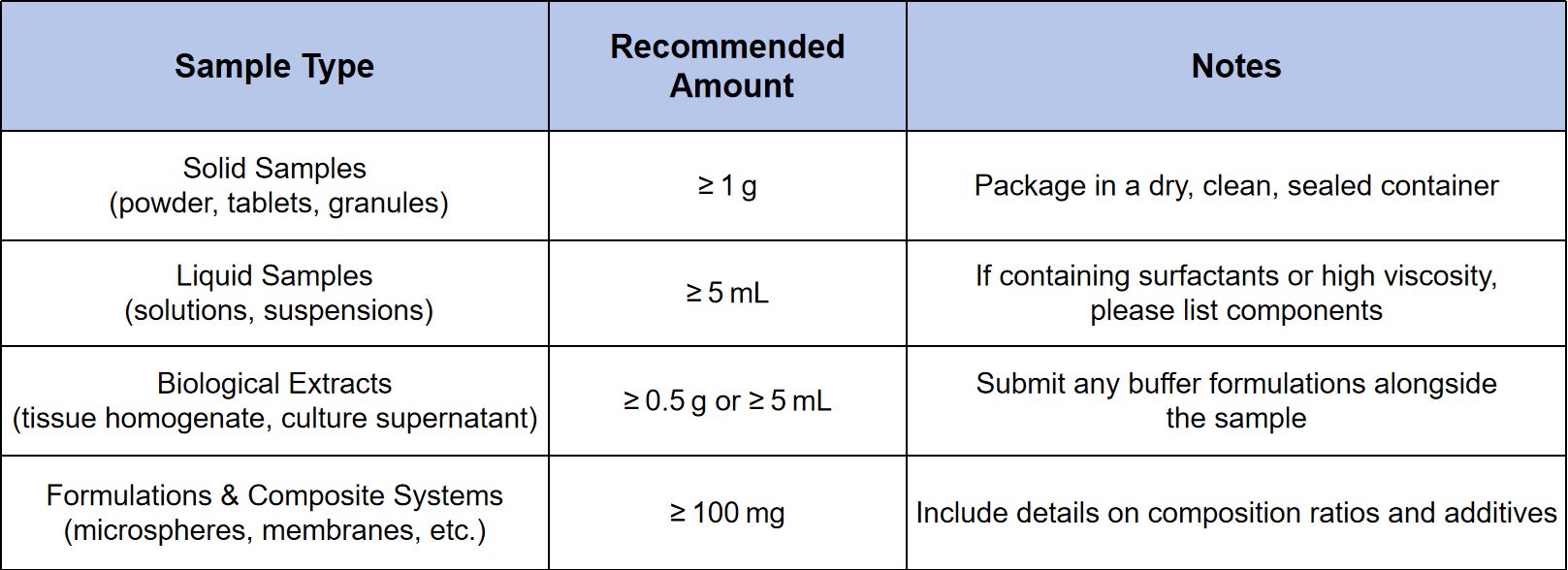
Storage & Transport Recommendations
For atypical sample types or if you are unsure about suitability, please contact our technical support team for personalized guidance.
Deliverables
✅ Quantitative Analysis Report (Units available: %w/w, ppm, mg/g, etc.)
✅ Raw Data: Includes complete spectra, thermogravimetric curves, or signal intensity graphs.
✅ Method Parameters
✅ PDF Format Electronic Report
How to order?







