Liposomes Structure Characterization Service | Cryo-EM
Liposomes structure characterization is a high-resolution structural analysis service based on cryogenic electron microscopy (Cryo-EM), specifically designed for observing and analyzing key features of liposomes, including their morphology, lamellarity, vesicle size, and bilayer membrane organization. This technique involves rapidly freezing liposome samples at liquid nitrogen temperature to prevent water crystallization and structural damage, followed by imaging under low-dose conditions, thereby faithfully preserving their native-state structure without the use of stains or chemical treatments.
Liposomes structure characterization service is widely applied in liposomal drug development, vaccine delivery systems, biomembrane modeling, and nanocarrier evaluation. Cryo-EM helps researchers assess the structural stability, drug-loading capacity, and release mechanisms of liposomes, providing a robust structural basis for the design and quality control of novel liposomal formulations.
Services at MtoZ Biolabs
Based on a high-end cryogenic electron microscopy platform, the liposomes structure characterization service based on Cryo-EM provided by MtoZ Biolabs enables rapid frozen and native-state fixation of liposome samples at ultra-low temperatures, achieving high-resolution imaging without the need for staining. This service delivers critical data on vesicle morphology, particle size distribution, number of lamellae, bilayer integrity, and internal structures of liposomes. Through structural analysis techniques, the acquired data ensures nanoscale spatial resolution and high structural fidelity, making it suitable for structural evaluation of novel liposomal drugs, carrier systems, and biomembrane models.
Analysis Workflow
1. Sample Vitrification
Liposome samples are rapidly cooled to liquid nitrogen temperature to form vitreous ice, preserving their native structure without damage.
2. Cryo-EM Image Acquisition
High-resolution, low-dose, multi-angle imaging is performed using a cryogenic transmission electron microscope to prevent sample damage.
3. Image Preprocessing and Particle Identification
Images are denoised, aligned, and screened for particle recognition, selecting high-quality data for structural analysis.
4. Structural Analysis
Structural analysis techniques are applied to evaluate key features such as particle size, lamellarity, and encapsulation status of liposomes.
5. Data and Report Delivery
Original images, structural models, and a professional analysis report are provided to support drug development, quality control, and scientific publication.
Service Advantages
1. Native-State Imaging with Structural Fidelity
Samples are immobilized under ultra-low temperature conditions, avoiding structural distortion caused by drying or staining and preserving the natural state of liposomes.
2. High Resolution
Utilizing advanced Cryo-EM platforms, the service enables clear visualization of lipid bilayers, lamellarity, and encapsulation status with nanometer-scale resolution.
3. Compatibility with Various Liposome Types
Applicable to structural analysis of unilamellar and multilamellar liposomes, as well as a wide range of drug delivery systems, meeting diverse research and development needs.
4. Stain-Free, Low-Dose Imaging
Eliminates artifacts from conventional staining and minimizes radiation damage through low-dose electron exposure, enhancing image quality and analytical accuracy.
Applications
1. Development of Liposomal Drug Formulations
The liposomes structure characterization service can be used to analyze the structural integrity, lamellarity, and particle size distribution of drug-loaded liposomes, helping to optimize formulation stability and release behavior.
2. Biomembrane Model Research
This service supports the simulation and analysis of native biomembrane structures, facilitating basic research in membrane protein behavior and drug mechanism studies.
3. Evaluation of Vaccine Delivery Systems
The liposomes structure characterization service can assess encapsulation efficiency, structural uniformity, and lipid organization in vaccine delivery, providing morphological evidence for vaccine design.
4. Quality Control and Batch Consistency Assessment
High-resolution structural comparison aids pharmaceutical companies in evaluating batch-to-batch consistency and improving manufacturing processes.
5. Characterization of Novel Lipid Materials
The liposomes structure characterization service can evaluate the structural performance of pH-sensitive, thermosensitive, or targeted liposomes, supporting the development of advanced nanocarriers.
Case Study
1. Imaging of Liposomes by Transmission Electron Microscopy
This study aims to explore the advantages of cryogenic electron microscopy (Cryo-EM) in liposome characterization, with a focus on its ability to preserve the native structure of liposomes and provide high-resolution images. The subjects are various types of liposome samples, including unilamellar and multilamellar structures. The authors rapidly froze the samples to form vitreous ice, avoiding structural deformation caused by conventional staining or drying, and conducted imaging under low-dose electron beam conditions. The results demonstrate that Cryo-EM accurately reveals key structural features such as spherical morphology, vesicle size, and membrane thickness of liposomes, with clear images and no artifacts. The study concludes that compared to conventional TEM methods such as negative staining, Cryo-EM offers superior structural fidelity without introducing external contrast agents, making it an ideal tool for investigating liposomal nanostructures and evaluating their stability and encapsulation performance, especially for environmentally sensitive liposome formulations.
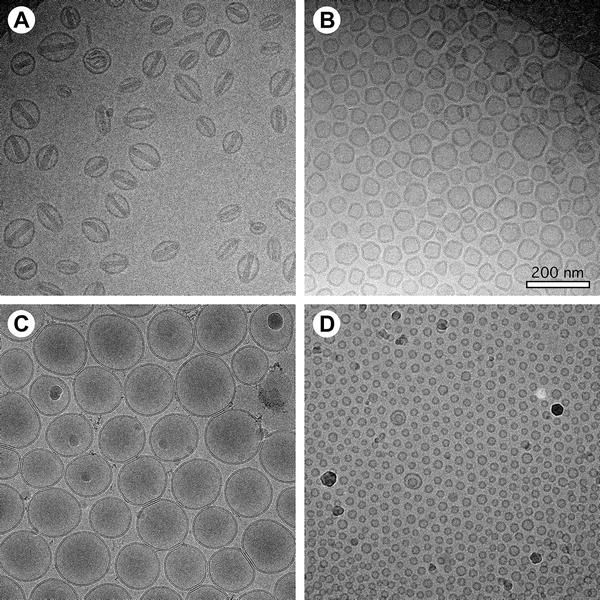
Baxa U. Method in Molecular Biology, 2017.
Figure 1. Examples of Cryo-EM Data of Liposome Preparations.
2. Biophysical Characterization of Polydisperse Liposomal Adjuvant Formulations
This study aims to use cryogenic electron microscopy (Cryo-EM) to characterize the structural properties of polydisperse liposomal adjuvant formulations, with a focus on analyzing membrane architecture and morphological features. The study targets two commonly used vaccine adjuvant liposome formulations: ALFlyo, which contains monophosphoryl lipid A (MPLA), and ALFQ, which contains both MPLA and the saponin QS21. The authors conducted Cryo-EM imaging on both formulations to investigate their vesicle structures and heterogeneity. The results showed that ALFlyo samples contained a large number of multilamellar and multivesicular liposomes with more complex structures, while ALFQ predominantly consisted of structurally uniform unilamellar liposomes, indicating that formulation components significantly influence liposome microstructure. The study concludes that Cryo-EM, as a high-resolution imaging technique, is effective for identifying and distinguishing liposome structural types, serving as a critical tool for evaluating the structural integrity and functional potential of liposomal adjuvants, with important implications for vaccine design and quality control.
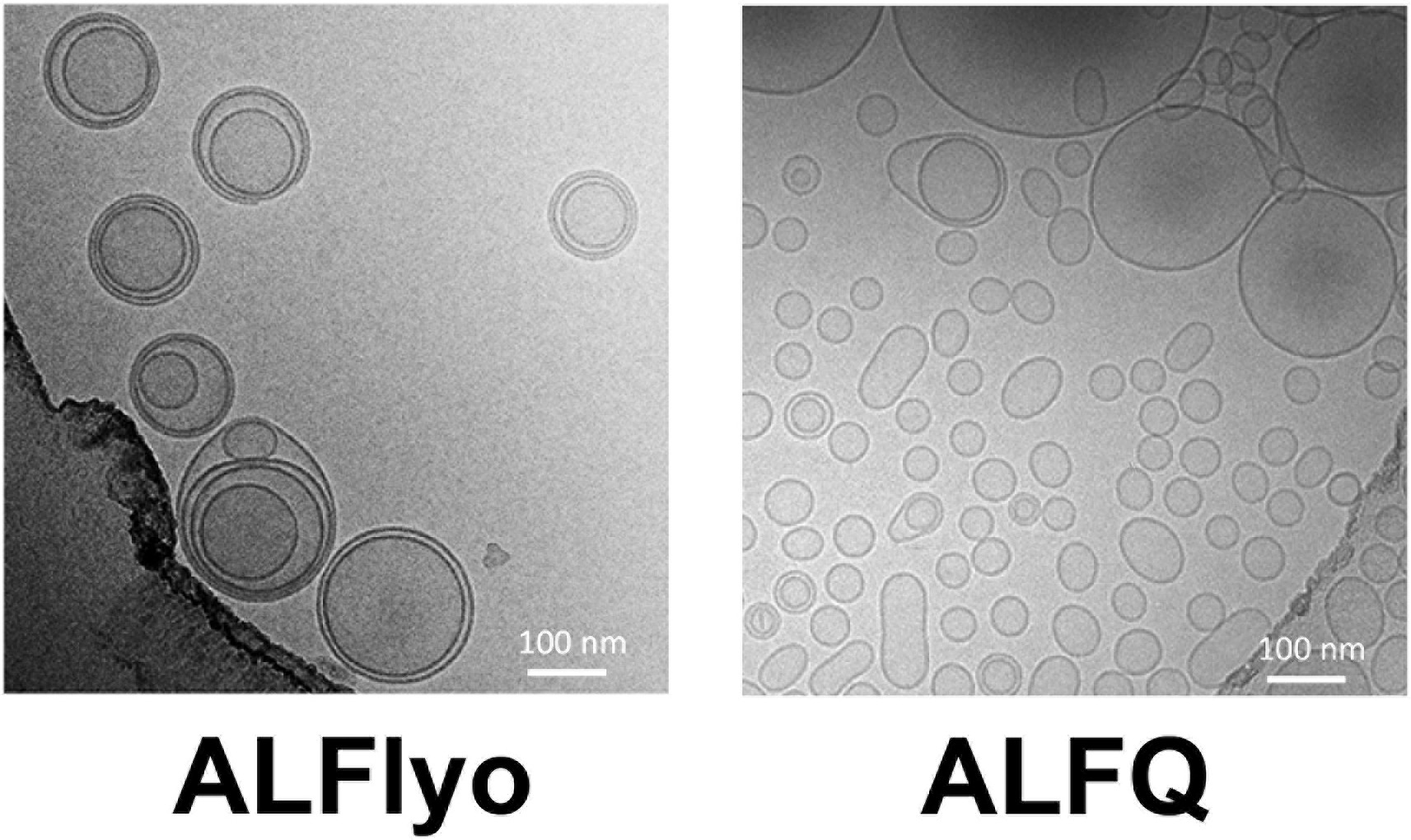
Singh, P. et al. Biochemical and Biophysical Research Communications, 2020.
Figure 2. Cryo-Electron Micrographs for (A) ALFlyo and (B) ALFQ.
FAQ
Q1: Why Is Cryo-EM Suitable for Liposome Structural Analysis?
A1: Cryo-EM enables rapid vitrification and imaging of liposomes under ultra-low temperature conditions, preventing structural deformation caused by drying or staining. It faithfully preserves the native vesicle architecture, membrane organization, and internal compartmentalization, making it especially suitable for structurally delicate and environmentally sensitive lipid systems.
Q2: Does Cryo-EM Damage the Structure of Liposomes?
A2: No. Cryo-EM uses rapid freezing and low-dose electron beam imaging, which causes minimal damage during the imaging process. It preserves the in situ structure of liposomes, making it ideal for native-state observation.
How to order?







