Lamellarity & Blebbing Analysis Service | Cryo-EM
Lamellarity & blebbing analysis is a high-resolution imaging and structural analysis technique based on cryogenic electron microscopy (Cryo-EM), specifically designed to evaluate the lamellarity and blebbing phenomena of membrane-based structures such as liposomes, extracellular vesicles (EVs), and exosomes. Lamellarity refers to the single- or multi-lamellar nature of membrane vesicles, while blebbing reflects the localized protrusions, deformations, or ruptures of the vesicle membrane. Cryo-EM rapidly freezes samples at liquid nitrogen temperature and images them under low-dose conditions, allowing for label-free, interference-free visualization of membrane layers, thickness, morphological variations, and overall integrity.
The lamellarity & blebbing analysis service is widely applied in liposome drug formulation consistency evaluation, extracellular vesicle quality control, development of novel nanocarriers, vesicle stability studies, and optimization of vaccine delivery systems. Through precise membrane structure analysis, this service provides critical structural evidence to support formulation process optimization, carrier design, improvement of drug delivery efficiency, and functional research.
Services at MtoZ Biolabs
Based on advanced cryogenic electron microscopy, MtoZ Biolabs offers the lamellarity & blebbing analysis service based on Cryo-EM, enabling rapid sample vitrification and high-resolution imaging under low-dose electron beam conditions without staining. This service accurately captures critical data on the lamellarity (single- or multi-lamellar structure), membrane thickness, and blebbing features of samples such as liposomes, extracellular vesicles (EVs), and exosomes. The results ensure nanometer-scale resolution and high structural fidelity, making the service ideal for quality control, structural evaluation, and functional research applications.
Analysis Workflow
1. Sample Vitrification
Rapidly cool liposome or vesicle samples to liquid nitrogen temperature to form amorphous ice, preserving the native membrane structure.
2. Low-Dose Imaging Acquisition
Perform low-dose, high-contrast two-dimensional imaging in a cryo-electron microscope to avoid radiation damage and ensure visualization of fine structural details.
3. Image Processing and Particle Identification
Denoise, align, and select high-quality membrane structures from raw images for subsequent analysis.
4. Lamellarity and Blebbing Feature Analysis
Quantify the proportion of single- and multilamellar vesicles, measure membrane thickness, and assess the presence of blebbing, protrusions, or membrane ruptures.
5. Data Compilation and Report Generation
Deliver raw images, quantitative analysis results, and structural feature reports to support formulation development, quality control, and functional mechanism studies.
Service Advantages
1. Accurate Preservation of Membrane Structure
Through rapid vitrification and low-dose imaging, the native membrane structure of vesicles is fully preserved, avoiding morphological artifacts caused by drying or staining.
2. High-Resolution Detail Capture
Utilizing advanced Cryo-EM platforms, nanometer-scale resolution is achieved, clearly revealing membrane lamellarity, thickness variations, and surface blebbing features.
3. Strong Quantitative Analysis Capability
Supports statistical analysis of key parameters such as single- and multilamellar vesicle ratios and blebbing frequency, providing standardized and quantifiable data outputs.
4. Comprehensive Structural Feature Assessment
Simultaneously delivers multidimensional information on membrane layering, blebbing phenomena, and vesicle integrity, offering a complete microstructural profile of the sample.
Applications
1. Liposome Formulation Quality Assessment
Lamellarity & blebbing analysis service can be used to evaluate the lamellar structure and membrane integrity of liposomal drug carriers, supporting formulation process optimization and quality control.
2. Nanocarrier Structural Validation
Cryo-EM enables the characterization of membrane structure and stability of novel nanoparticles or synthetic vesicles, ensuring consistent performance in delivery systems.
3. Extracellular Vesicle Structural Research
Lamellarity & blebbing analysis service can assess the single- or multilamellar nature and membrane blebbing features of EVs and exosomes, supporting functional mechanism exploration and biomarker development.
4. Vesicle Stability and Storage Studies
By monitoring changes in membrane layers and blebbing, the service evaluates vesicle structural stability and preservation performance under various treatment conditions.
Case Study
1. Analysis of the Formation of the Human Skin Barrier Using Cryo-Electron Microscopy and EM Simulations
This study utilized cryo-electron microscopy (Cryo-EM) combined with electron microscopy simulation techniques to analyze lipid phase transitions during human skin barrier formation. The research focused on the secretory systems of human epidermal cells and intercellular lipid layers, using in situ Cryo-EM imaging and molecular dynamics simulations. Results showed that lipids initially existed in a bicontinuous cubic phase and subsequently reorganized into a highly ordered lamellar structure, with localized membrane blebbing and rearrangement observed during the transition. Simulation data were consistent with Cryo-EM images, revealing that deglycosylation and dehydration played key roles in the structural transformation. The study demonstrated that skin barrier formation involves an orderly cubic-to-lamellar phase transition, highlighting Cryo-EM as a critical tool for elucidating lipid membrane reorganization and barrier function establishment.
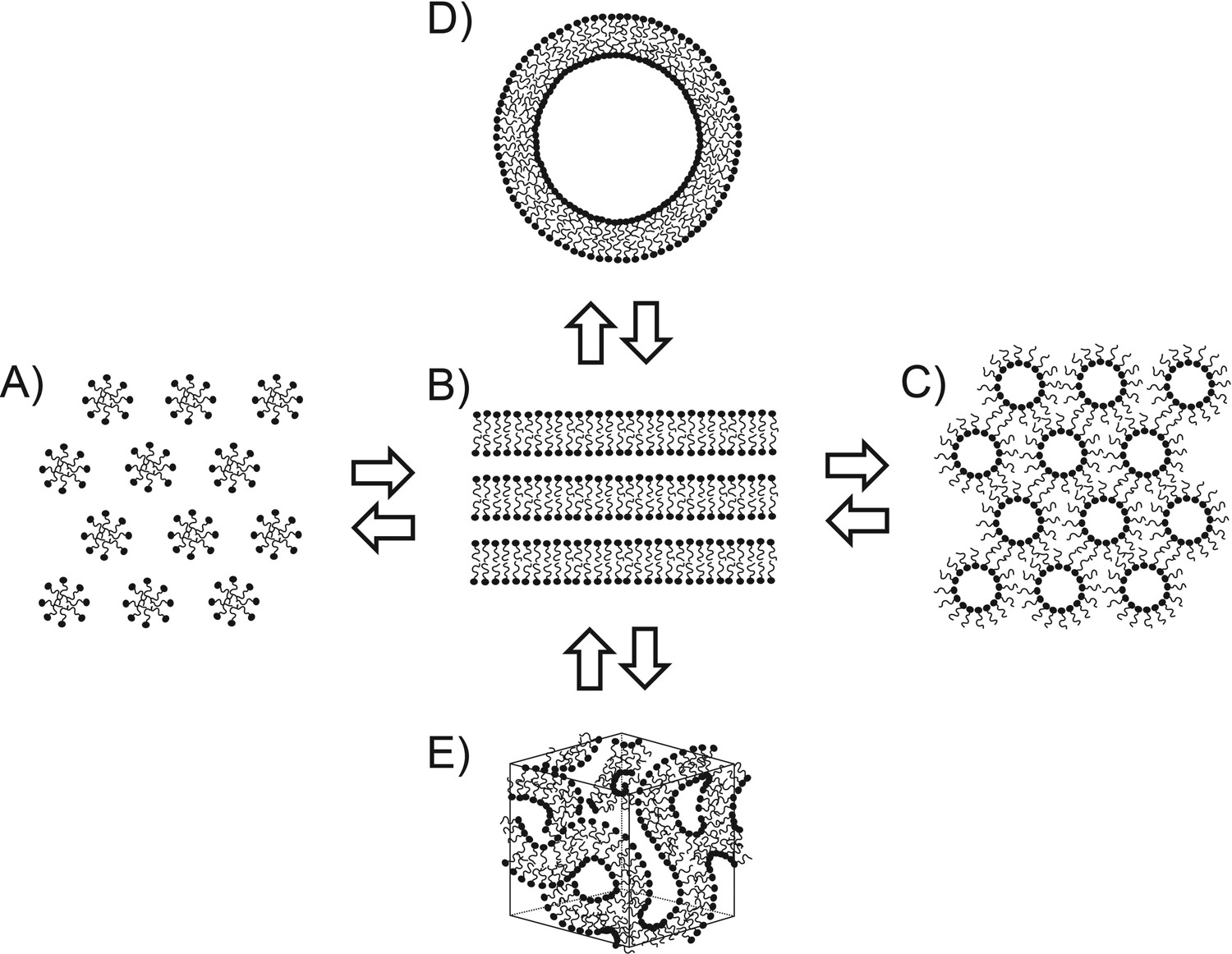
Narangifard, A. et al. Experimental Cell Research, 2018.
Figure 1. Schematic View of Tentative Models for the Formation of the Skin's Permeability Barrier.
2. Phage-Mediated Explosive Cell Lysis Induces the Formation of a Different Type of O-IMV in Shewanella vesiculosa M7T
This study utilized cryo-electron microscopy (Cryo-EM) combined with flow cytometry to analyze the membrane vesicles (MVs) formed by the psychrophilic bacterium Shewanella vesiculosa M7T at different growth stages. The research focused on naturally secreted outer membrane vesicles (OMVs) and outer-inner membrane vesicles (O-IMVs). Cryo-EM imaging revealed that O-IMVs possess distinct double-membrane structures accompanied by localized membrane blebbing. The study found that, in addition to conventional budding-derived O-IMVs, phage-mediated explosive cell lysis could generate structurally distinct explosive O-IMVs (EO-IMVs), which exhibit significant differences in membrane continuity and morphology compared to regular O-IMVs. The results indicate that Shewanella vesiculosa M7T employs diverse vesicle formation mechanisms involving membrane remodeling and blebbing, and that Cryo-EM provides critical support for elucidating the lamellarity and dynamic changes of vesicles.
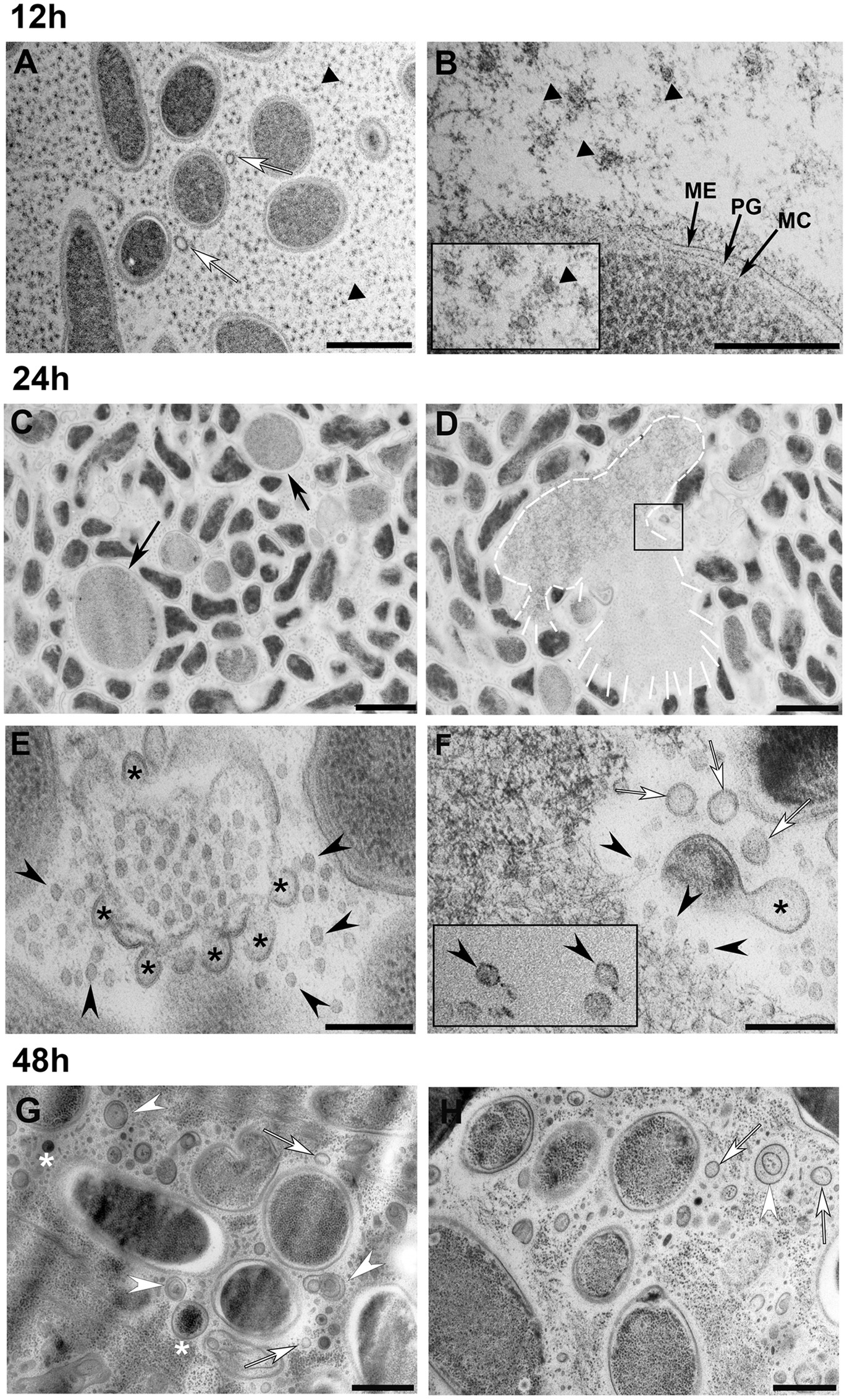
Baeza, N. et al. Frontiers in Microbiology, 2021.
Figure 2. Transmission Electron Microscopy Observation of S. vesiculosa M7T Cells and MVs at Different Times of Growth.
FAQ
Q1: Can this Service Distinguish between Unilamellar and Multilamellar Vesicles?
A1: Yes. High-resolution Cryo-EM imaging enables clear identification of the number of membrane layers, allowing accurate differentiation between unilamellar, bilamellar, and multilamellar structures, along with statistical analysis.
Q2: Can Membrane Blebbing Phenomena Be Observed in Vesicles?
A2: Yes. Cryo-EM can capture subtle surface protrusions, ruptures, or blebbing on vesicle membranes, supporting detailed analysis of membrane stability and vesicle integrity.
How to order?







