In-situ Infrared Spectroscopy Analytical Service
In-situ infrared spectroscopy analytical is an analytical technique used to monitor molecular structure changes of samples in real time under specific reaction or treatment environments. Based on infrared spectroscopy, it records the infrared absorption spectra of molecules during chemical reactions or physical changes to identify variations in specific functional groups, thereby inferring reaction pathways, intermediate formation, and decomposition behavior. This method enables in-situ data acquisition within the reaction system without interfering with the system itself and features non-destructiveness, high sensitivity, and high temporal resolution.
In-situ infrared spectroscopy analytical service is widely applied across various research and industrial fields, including heterogeneous catalysis, electrocatalysis and electrochemical reaction mechanism analysis, gas adsorption and desorption studies, polymerization monitoring, surface functional group transformation analysis, and pharmaceutical polymorphic conversion and stability evaluation. It is especially suitable for investigating reaction dynamics, short-lived intermediates, and interfacial reaction behavior, providing critical data support for material optimization, reaction mechanism elucidation, and process scale-up.
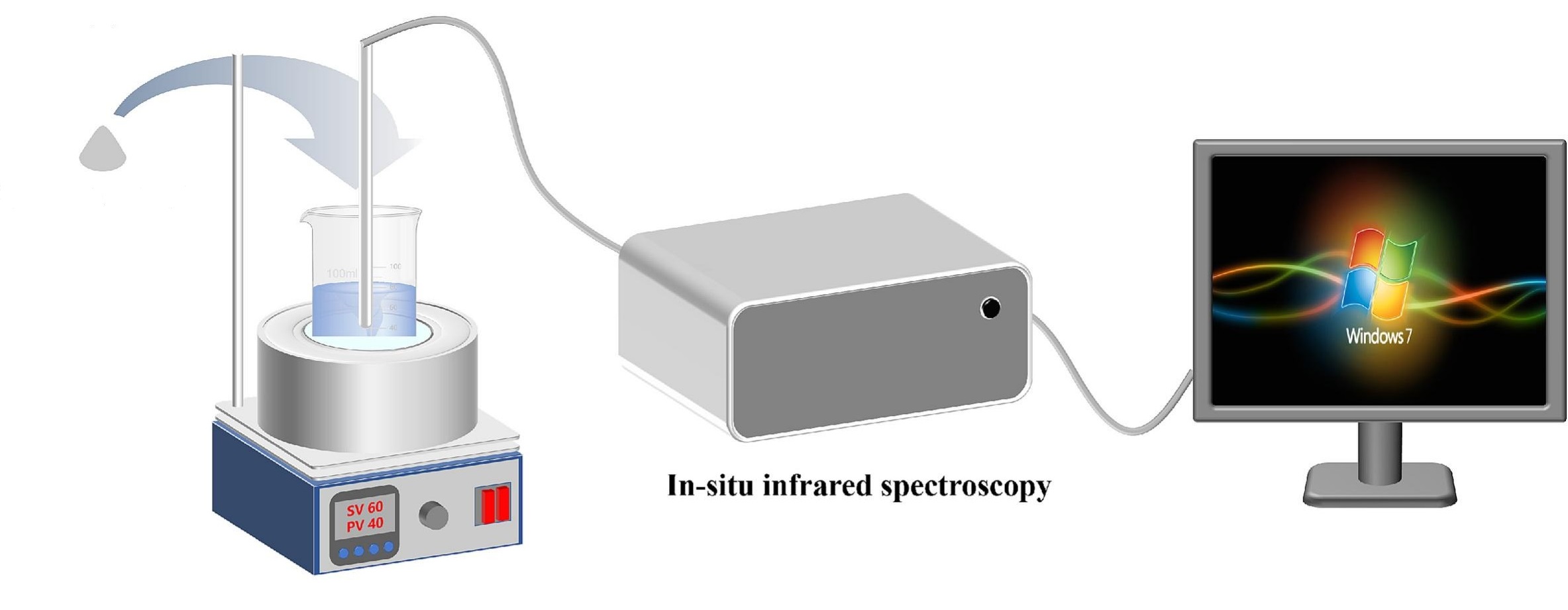
Sun, X. et al. Desalination, 2025.
Figure 1. In-situ Infrared Spectroscopy Experimental Device.
Services at MtoZ Biolabs
Based on in-situ infrared spectroscopy technology combined with advanced analytical theory, MtoZ Biolabs provides in-situ infrared spectroscopy analytical service capable of real-time monitoring of infrared signals in gas, liquid, and solid multiphase reaction systems. This service integrates temperature control, atmosphere regulation, and online acquisition systems to precisely track the evolution of functional groups during reactions, ensuring high temporal resolution and high signal-to-noise ratio spectral data. It is well-suited for reaction mechanism elucidation, intermediate identification, and the investigation of dynamic behaviors on material surfaces.
Analysis Workflow
1. Sample Preparation and Loading
Place the sample to be tested into a dedicated in-situ IR reaction cell, ensuring good optical transparency and reactive compatibility. Choose appropriate supports or catalysts based on the research objectives.
2. Reaction Condition Setup
Adjust experimental conditions such as temperature, atmosphere (e.g., H₂, O₂, CO₂), humidity, or light exposure to simulate the actual reaction environment, and activate the reaction control system.
3. Real-Time Infrared Spectral Acquisition
Continuously collect infrared absorption changes of functional groups on the sample surface or in the gas phase during the reaction using an infrared spectrometer, recording dynamic changes at critical time points.
4. Data Analysis and Processing
Identify characteristic peaks, analyze variation trends, and monitor intermediates from the acquired spectra. Combine quantitative or semi-quantitative approaches to interpret reaction mechanisms and catalytic processes.
5. Result Output
Deliver a comprehensive report including experimental conditions, IR spectra, functional group identification, and mechanistic analysis, supporting research validation and catalyst performance optimization.
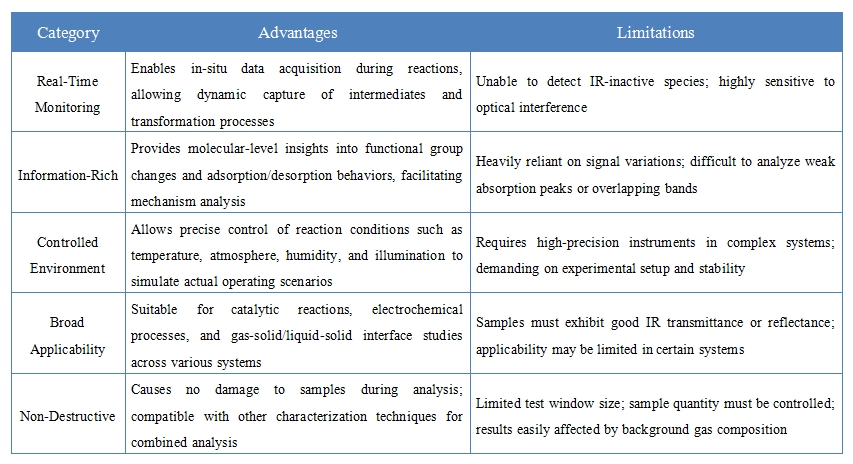
Advantages and Limitations
Applications
1. Catalytic Reaction Mechanism Investigation
The in-situ infrared spectroscopy analytical service can be used to monitor functional group changes of reactants, intermediates, and products during catalytic reactions, revealing adsorption, activation, and transformation pathways. It is widely applied in heterogeneous catalysis, photocatalysis, and electrocatalysis research.
2. Electrochemical Interface Analysis
By integrating with an in-situ electrochemical cell, this service enables the study of dynamic changes on electrode surfaces during reactions such as oxygen reduction, hydrogen evolution, and carbon dioxide reduction, helping elucidate interfacial structural evolution and reaction mechanisms.
3. Surface Adsorption/Desorption Behavior Studies
In-situ infrared spectroscopy analytical service is ideal for evaluating the real-time adsorption states and bonding modes of gases or small molecules on material surfaces, applicable to adsorbent screening, separation membranes, and sensing materials.
4. Polymorphic Transformation and Stability Evaluation in Pharmaceuticals
In drug development, the service can monitor polymorphic transitions, hydration processes, or the stability of functional groups, aiding in the understanding of structural changes under different environmental conditions.
5. Reaction Monitoring for Environmental and Energy Materials
In-situ infrared spectroscopy analytical service is extensively used to track reaction behaviors in fuel cells, photothermal conversion materials, and CO₂ capture systems, providing support for performance optimization and environmental adaptation of functional materials.
Case Study
1. Water Dimer in CCl₄ Investigated by In-situ Infrared Spectroscopy and Computational Analysis
The aim of this study is to investigate the formation and structural characteristics of water dimers in carbon tetrachloride (CCl₄), focusing on hydrogen-bonded aggregates formed by trace amounts of water molecules in CCl₄ solution. In-situ infrared (IR) spectroscopy combined with density functional theory (DFT) calculations was employed to systematically analyze hydrogen bond formation between water molecules, as well as their stability and IR absorption features in solution. Experimental results show that water molecules readily form stable dimers in CCl₄, with the O–H stretching vibrations exhibiting a pronounced red shift in the IR spectra, which aligns well with theoretical predictions. The study concludes that in-situ IR spectroscopy, combined with computational modeling, is an effective approach to reveal molecular association behavior in weakly interacting systems, providing a reliable method and theoretical basis for understanding hydrogen bond network formation in non-polar solvents.
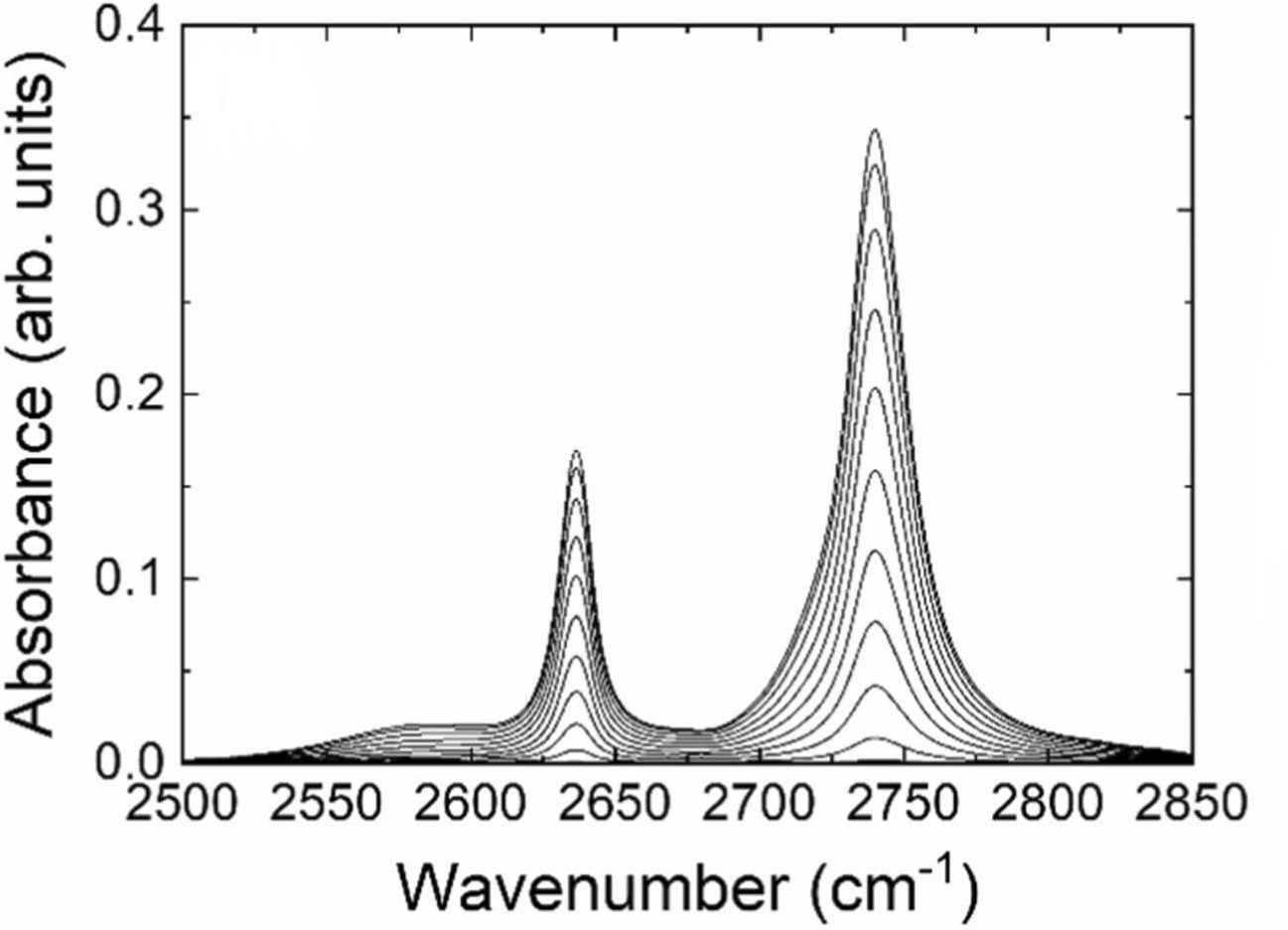
Lee, J. et al. Vibrational Spectroscopy, 2024.
Figure 2. Time-Series IR Spectra for In-situ Inclusion of D2O in CHCl3.
FAQ
Q1: What Is the Difference Between In-situ Infrared and Conventional Infrared Spectroscopy?
A1: Conventional infrared spectroscopy is typically used for analyzing the structure of static samples, while in-situ infrared spectroscopy enables real-time monitoring of functional group changes during a reaction. It captures intermediates and transformation pathways throughout the dynamic reaction process, making it ideal for studying reaction mechanisms and catalytic processes.
Q2: What Key Information Can Be Obtained from the Analysis Results?
A2: The analysis can reveal changes in infrared absorption of reactants, intermediates, and products during the reaction, allowing for the identification of functional group transformations, reaction pathways, and adsorption behaviors. This information supports mechanistic studies and material optimization.
How to order?







