Immunomodulating Multi-antibody-modified Exosome Service
Immunomodulating Multi-antibody-modified Exosome Service is designed to integrate multiple immunomodulatory antibodies onto the surface of exosomes, creating an innovative therapeutic tool with enhanced immune-regulating capabilities and targeting specificity. MtoZ Biolabs offers comprehensive services encompassing exosome isolation and purification, antibody modification, and functional validation, supporting researchers in advancing exosome-based immunotherapy studies and applications.
As natural nanoscale vesicles for intercellular communication, exosomes have been widely applied in drug delivery, vaccine development, and immunotherapy. With the advancement of multi-specific antibody technologies, modifying exosomes with multiple immunomodulatory antibodies (e.g., PD-L1, CD3) has emerged as a powerful approach to enhance their targeting ability, regulatory precision, and therapeutic efficacy. By directionally modifying exosomal surfaces with various immunoregulatory antibody molecules, it is possible to achieve simultaneous blockade of immune suppression signals, activation of effector T cells, enhancement of co-stimulatory pathways, or multi-site immune modulation, providing a multifaceted strategy for next-generation immunotherapies.
Fan Y. et al. Cancer Immunotherapy. 2021.
Leveraging an advanced exosome engineering platform, MtoZ Biolabs offers Immunomodulating Multi-antibody-modified Exosome Service enabling precise modulation of key immune pathways—such as immune checkpoints, T cell activation receptors, and co-stimulatory molecules—through genetic engineering or surface conjugation strategies. This service supports research in tumor immunotherapy, cell-based therapies, and personalized immune intervention by providing highly customized, multi-target exosomal delivery systems.
Analysis Workflow
MtoZ Biolabs offers end-to-end customization for Immunomodulating Multi-antibody-modified Exosome Service, covering everything from surface engineering to functional validation:
1. Multi-Specific Antibody Modification & Exosome Isolation and Purification
Genetic engineering method: Transfect plasmids encoding multi-specific antibody-membrane protein fusion genes into exosome-producing cells, enabling the secreted exosomes to display multi-specific antibodies on their surface. Exosomes are then isolated and purified from cell culture supernatants or biological fluids.
Chemical modification method: Isolate and purify exosomes from culture media or body fluids. Use click chemistry or PEG-mediated approaches to directly conjugate pre-prepared multi-specific antibodies onto the exosome membrane surface.
2. Characterization of Modified Exosomes
Utilize techniques such as Transmission Electron Microscopy (TEM), Nanoparticle Tracking Analysis (NTA), Dynamic Light Scattering (DLS), and Western blot to comprehensively evaluate particle size, morphology, surface charge, and antibody display, ensuring quality and consistency.
3. Functional Validation (optional)
Assess the biological functions of modified exosomes in vitro and in vivo, including target cell binding ability and immune modulation effects.
Applications
Enhanced Tumor Immunotherapy: Simultaneous blockade of immune checkpoints and activation of T cells for improved anti-tumor response.
Supportive Vector for CAR-T Therapies: Provide an immune-supportive microenvironment for CAR-T expansion and persistence.
Immunomodulation in Chronic Viral Infections: Improve the persistence and specificity of T cell effects.
Autoimmune and Immune Tolerance Studies: Design suppressive antibody-modified exosomes for negative immune regulation models.
Vaccine Adjuvant System Development: Enhances antigen-specific cellular immune responses.
Service Advantages
Dual/triple antibody surface display with precise spatial control.
A variety of modification methods are available for flexible selection to suit different systems.
Directed expression and functional site retention are taken into account.
Comprehensive quality control: size, surface markers, targeting capacity, binding activity.
FAQ
Q. Can you co-modify exosomes with two or three antibodies simultaneously? Is it possible to control the relative expression or ratio of each antibody?
Yes. We support dual or triple antibody co-modification on the exosome surface. Using a gene engineering approach, plasmids encoding each antibody fused with membrane-anchoring proteins (e.g., CD63, Lamp2b) are co-transfected into exosome-producing cells. The expression ratio of each antibody can be fine-tuned by adjusting the plasmid ratios. Western blot and flow cytometry are used for quantitative validation and optimization.
Q. What types of immunomodulatory antibodies are supported?
MtoZ Biolabs supports a wide range of immunomodulatory antibodies including imune checkpoint targets(such as PD-L1, PD-1), T cell activation targets (such as CD3, CD28), co-stimulatory molecules (such as OX40L, 4-1BBL). Clients may provide custom antibody sequences or targets. We offer full-service antibody cloning, expression, membrane-anchoring design, and functional validation to ensure that the engineered exosomes exhibit stable and specific immune regulatory activity.
Case Study
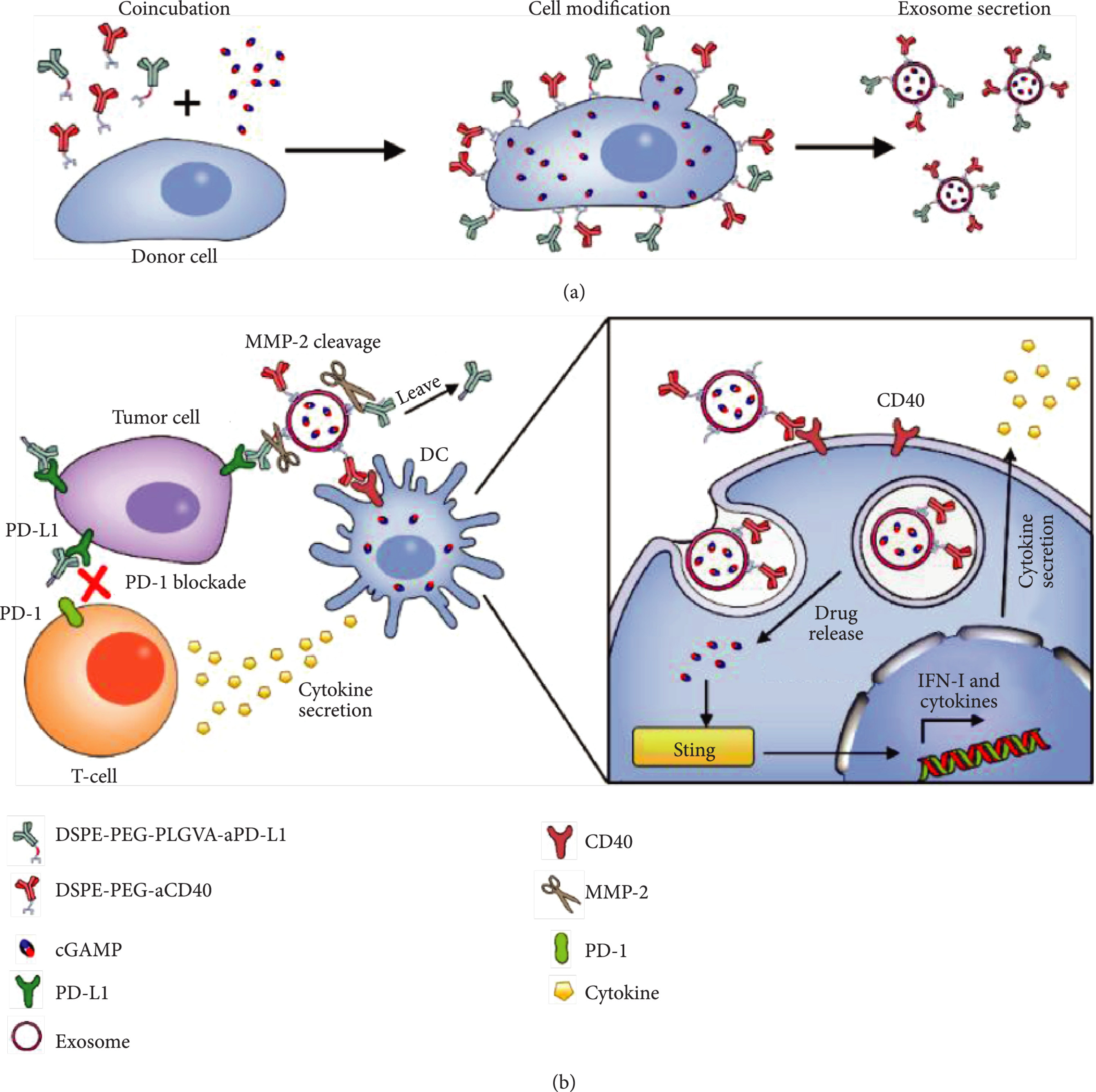
In this study, exosomes were co-modified with anti-PD-L1 and anti-CD40 antibodies. Anti-PD-L1 blocked tumor immune evasion, while anti-CD40 activated dendritic cells, creating a dual mechanism of immune activation and checkpoint inhibition. Additionally, the exosome core was loaded with the STING agonist cGAMP to enhance innate immunity. Results showed the dual-targeted exosome system significantly improved T-cell activation and tumor cell clearance in vitro and effectively suppressed tumor growth in a murine model, demonstrating the strong potential of multi-specific antibody-modified exosomes in combination immunotherapy.
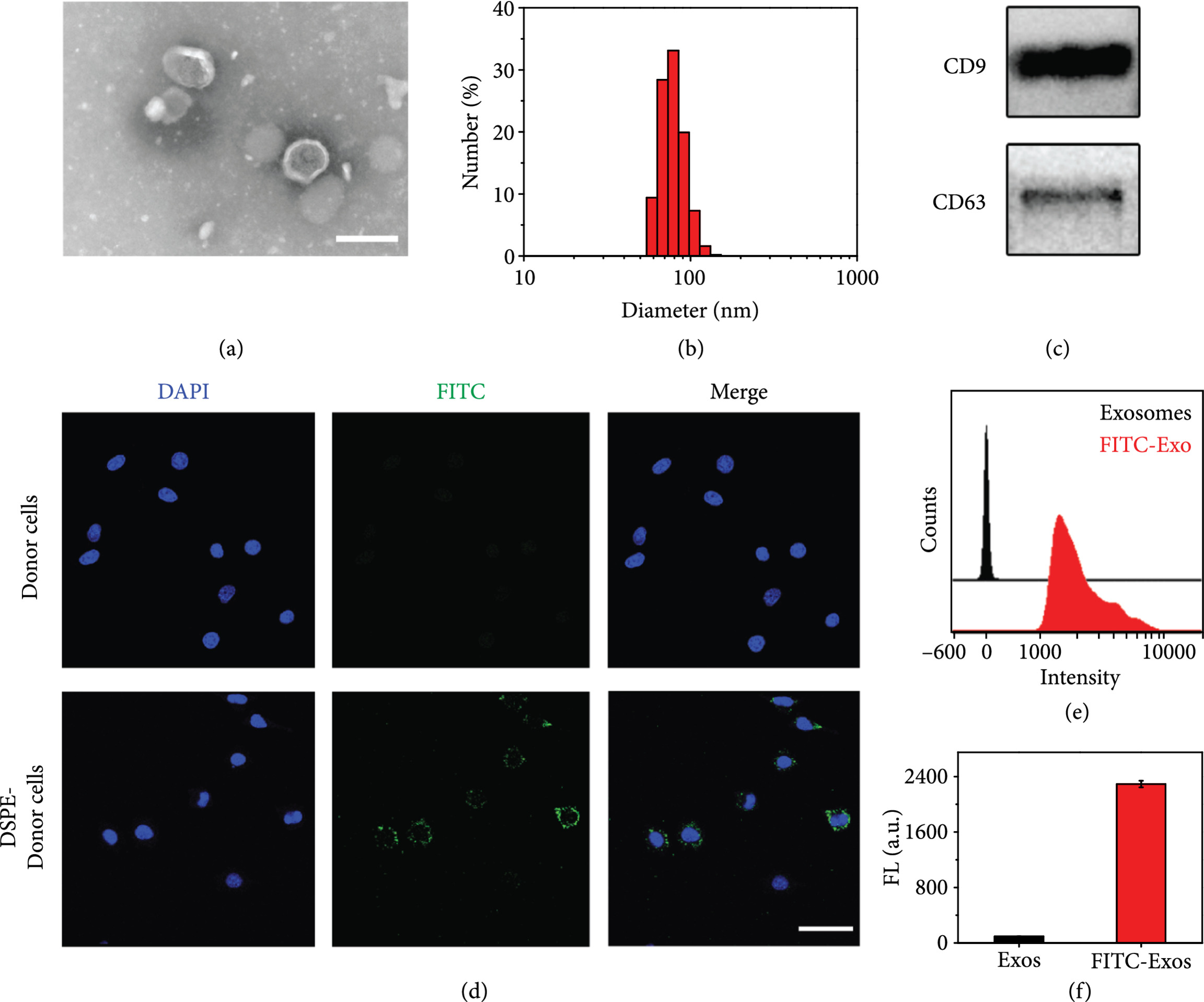
Fan Y. et al. Cancer Immunotherapy. 2021.
How to order?







