Human Papillomavirus (HPV) Structure Characterization Service | Cryo-EM
HPV is a non-enveloped double-stranded DNA virus with a capsid composed of L1 and L2 proteins arranged in icosahedral symmetry. Human papillomavirus (HPV) structure characterization is a high-resolution imaging technique based on cryogenic electron microscopy (Cryo-EM), specifically designed to analyze the three-dimensional structure and assembly features of human papillomavirus (HPV). Cryo-EM rapidly freezes HPV samples to liquid nitrogen temperature, preventing structural distortion caused by drying or staining, and captures images under low-dose electron beams, thereby preserving the native conformation of the virus particles and achieving nanometer to sub-nanometer resolution.
The human papillomavirus (HPV) structure characterization service is suitable for observing the spatial arrangement of HPV capsid proteins, evaluating assembly integrity, and distinguishing between empty and full particles. It supports 3D reconstruction and detailed structural annotation. This service is widely applied in HPV vaccine design, capsid stability assessment, genome packaging mechanism studies, and viral vector development, providing essential structural insights for antiviral drug research, vaccine optimization, and exploration of viral functional mechanisms.
Services at MtoZ Biolabs
Based on an advanced cryogenic electron microscopy platform, MtoZ Biolabs provides a human papillomavirus (HPV) structure characterization service based on Cryo-EM, which enables the rapid freezing of HPV samples to liquid nitrogen temperature, high-resolution, label-free imaging is achieved with a low-dose electron beam. This service delivers detailed information on the 3D structure of HPV particles, capsid symmetry, assembly state, and the ratio of empty to full particles. It ensures nanometer-level resolution and structural fidelity, making it ideal for applications in vaccine structure optimization, viral sample evaluation, and vector development.
Analysis Workflow
1. Sample Freezing Fixation
Rapidly cool HPV samples to liquid nitrogen temperature to form vitreous ice, preserving the native structure of viral particles and preventing denaturation or aggregation.
2. Low-Dose Image Acquisition
Capture high-contrast, low-dose 2D images using a cryogenic transmission electron microscope to ensure image quality and structural integrity.
3. Image Processing and Particle Selection
Denoise, align, and filter raw images to extract high-quality viral particles for further analysis.
4. 3D Structure Reconstruction
Reconstruct three-dimensional models from selected particles to analyze the spatial arrangement, symmetry, and assembly characteristics of the HPV capsid.
5. Data Output and Report Generation
Deliver raw images, 3D structural models, and detailed analysis reports to support vaccine development, quality assessment, and scientific publication.
Service Advantages
1. Preservation of Native Structure
Rapid freezing stabilizes the sample without staining or drying, accurately maintaining the native conformation of HPV particles.
2. High-Resolution Imaging
Utilizing advanced Cryo-EM platforms, the service enables nanometer to sub-nanometer level 3D structural reconstruction to precisely visualize capsid protein arrangement and symmetry.
3. Accurate Differentiation of Empty and Full Particles
By analyzing image density and structural reconstruction, the method distinguishes empty capsids from genome-filled particles, supporting evaluation of packaging efficiency and product quality.
4. Improve Structural Research Efficiency
Standardized image processing and three-dimensional reconstruction processes are provided to greatly shorten the structural analysis cycle and speed up the development process of vaccines and viral vectors.
Applications
1. Vaccine Development and Structural Optimization
The human papillomavirus (HPV) structure characterization service can be used to assess the structural consistency of HPV particles produced under different expression systems or purification processes, guiding vaccine formulation optimization and quality enhancement.
2. Capsid Assembly Mechanism Study
Through 3D structural reconstruction, the service reveals the spatial arrangement of L1 and L2 proteins within viral particles, aiding in the understanding of HPV assembly and capsid stability.
3. Viral Vector Development and Validation
The human papillomavirus (HPV) structure characterization service supports the structural validation of engineered HPV particles or virus-like particles (VLPs), facilitating their application in gene delivery and nanomaterial development.
4. Packaging Efficiency and Particle Integrity Analysis
Cryo-EM enables precise differentiation between empty and full HPV particles, allowing for quantitative assessment of packaging efficiency and providing structural data for vaccine efficacy and formulation process optimization.
Case Study
1. Characterization of an Escherichia Coli-Derived Human Papillomavirus Type 16 and 18 Bivalent Vaccine
This study utilized cryo-electron microscopy (Cryo-EM) to perform structural characterization of HPV type 16 and 18 virus-like particles (VLPs) produced from Escherichia coli, aiming to evaluate their morphological stability and structural consistency. The VLPs were assembled from recombinant L1 proteins expressed using a non-fusion, soluble expression system and exhibited a T=7 icosahedral symmetry. Cryo-EM 3D reconstruction revealed that the size and symmetry of the vaccine VLPs were highly consistent with native HPV particles, with well-maintained structural integrity. Batch-to-batch comparison further confirmed the reproducibility of structural features across different production scales. The findings demonstrate that Cryo-EM is a reliable method for HPV vaccine structural validation, providing essential structural evidence for quality control and industrial-scale development of E. coli-derived vaccines.
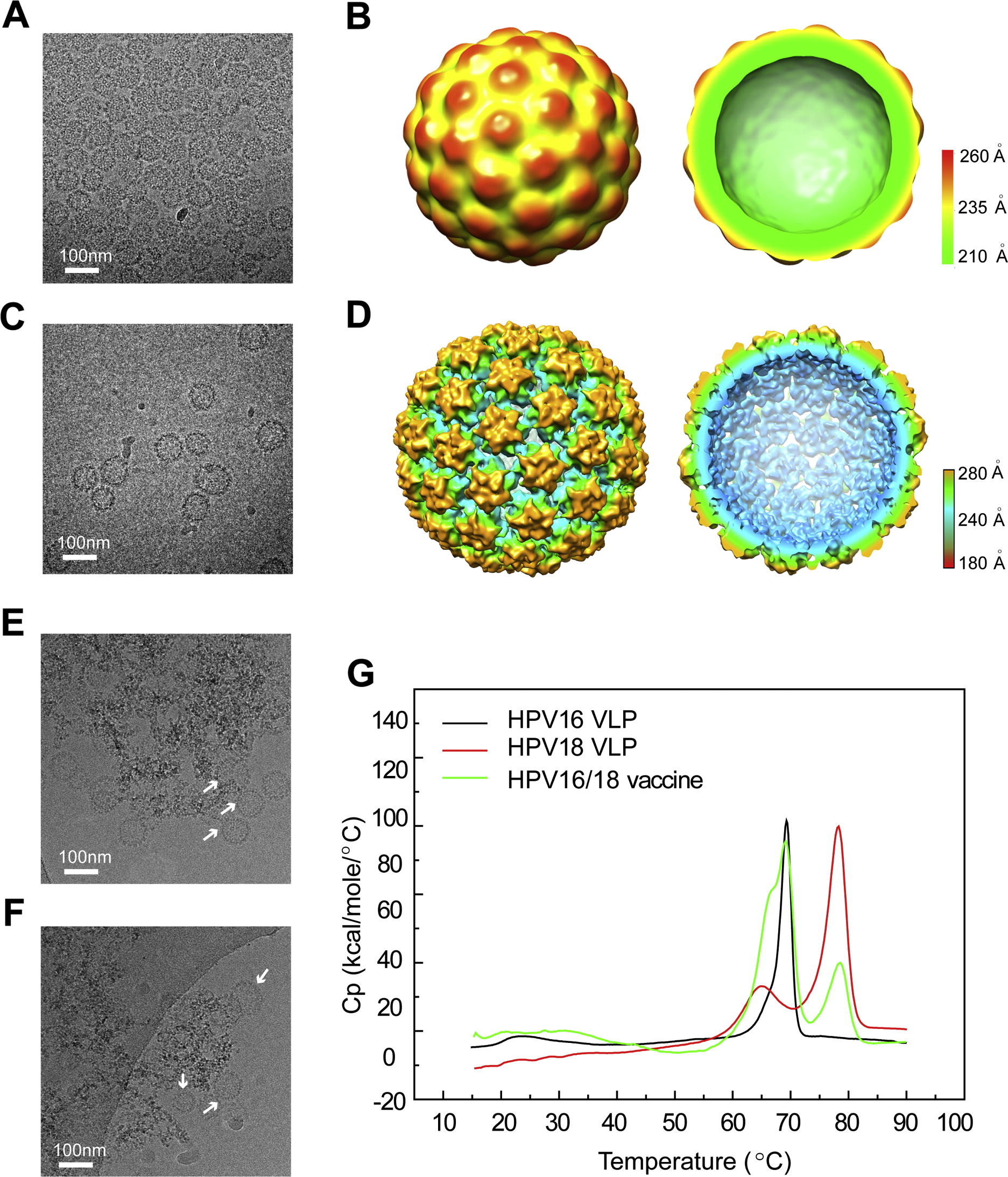
Gu, Y. et al. Vaccine, 2017.
Figure 1. Structural and Thermal Characterization of HPV16 and 18 L1 Virus-Like Particles (VLPs) in Solution and of VLPs Absorbed with Aluminum.
2. The U4 Antibody Epitope on Human Papillomavirus 16 Identified by Cryo-electron Microscopy
This study employed cryo-electron microscopy (Cryo-EM) to determine the 3D structure of the human papillomavirus type 16 (HPV16) pseudovirus in complex with the monoclonal antibody H16.U4, aiming to identify the conformationally dependent neutralizing epitope of H16.U4. The target was HPV16 pseudovirus particles, and through Cryo-EM imaging combined with atomic model fitting, the antibody was found to primarily bind to the C-terminal arm region of the L1 protein, specifically at conformational epitopes near the fivefold vertex. Although there are theoretically 360 potential binding sites, H16.U4 Fab selectively binds only to epitopes surrounding the pentameric vertex, indicating strong local conformational specificity. The study further demonstrated that H16.U4 exerts its neutralizing effect by blocking the initial interaction between the virus and host cell receptors. This work highlights the essential role of Cryo-EM in resolving conformational epitopes on viral capsids and provides important structural insights for HPV vaccine design and the study of viral infection mechanisms.
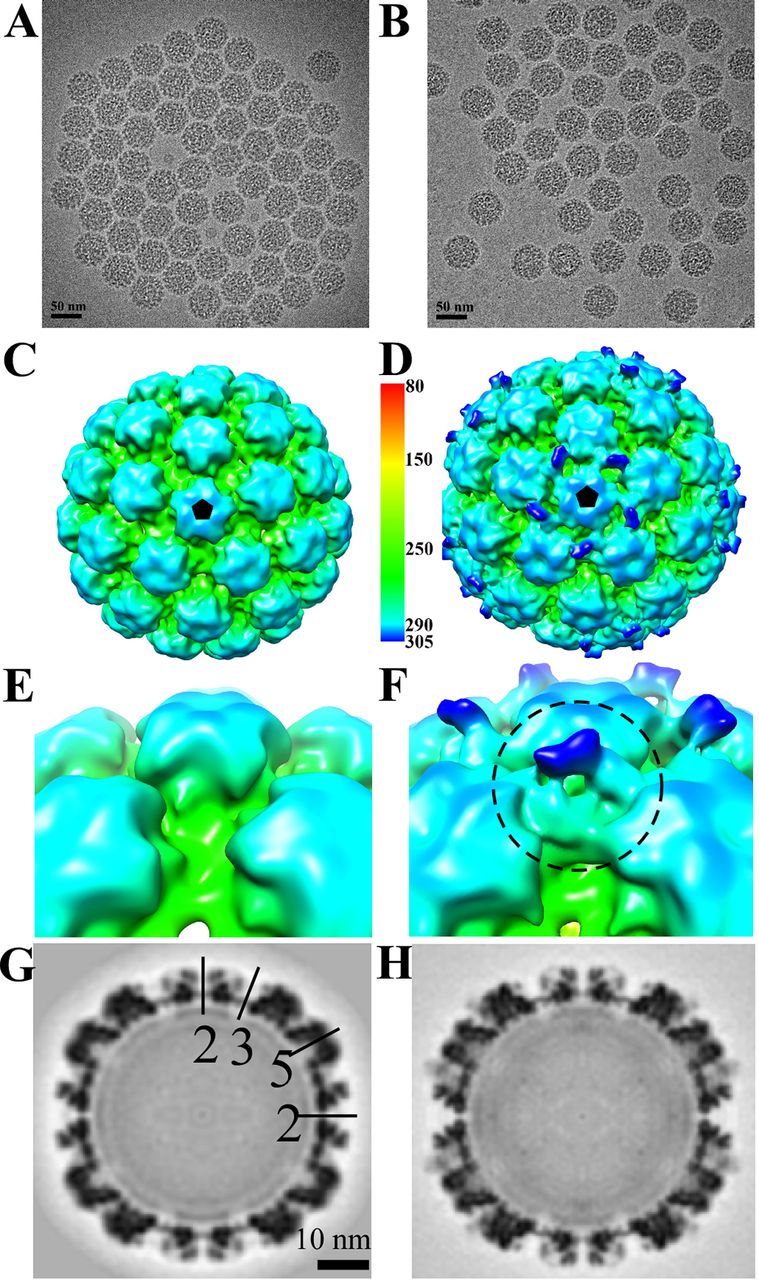
Guan J. et al. Journal of Virology, 2015.
Figure 2. Cryo-EM Reconstructions of HPV16 and HPV16-Fab Complexes.
FAQ
Q1: Will Cryo-EM Damage the Structure of HPV Particles?
A1: No. Cryo-EM uses low-dose electron beam imaging combined with rapid freezing, allowing for preservation of HPV particles in their native state without the need for staining or drying, thereby avoiding conformational disruption.
Q2: Can this Service Visualize the Structural Distribution of L1 and L2 Proteins?
A2: Yes. When resolution is sufficient, Cryo-EM can resolve the symmetrical arrangement of L1 proteins in the HPV capsid and, under certain conditions, reveal the localization of L2 proteins, supporting in-depth studies of viral assembly mechanisms.
How to order?







