High-Accuracy Antibody Sequencing Using De Novo Techniques
-
Full-length antibody sequence determination (heavy and light chains)
-
High-resolution validation of CDR region coverage
-
Comprehensive mapping of antibody modifications (e.g., glycosylation, oxidation)
-
Analysis of samples containing homologous antibody mixtures
Introduction: Why Is Antibody Sequencing Becoming Increasingly Important?
Antibodies, as key effectors of the adaptive immune system, exhibit remarkable diversity and specificity that make them indispensable in basic research, diagnostic development, and biopharmaceutical applications. From the generation of monoclonal antibodies to the evaluation of novel vaccines and the elucidation of tumor immunotherapy mechanisms, acquiring complete antibody sequence information is a critical initial step.
Conventional antibody sequencing approaches typically rely on known sequence databases or mRNA extracted from antibody-producing cells, which limits their applicability to natural antibodies, polyclonal mixtures, or rare antibodies present in clinical samples. In this context, mass spectrometry-based De Novo techniques for antibody sequencing have emerged as a powerful and increasingly important tool in antibody discovery and characterization.
What Are De Novo Techniques in Antibody Sequencing?
De Novo sequencing refers to the process of directly deducing protein sequences from mass spectrometry data without relying on existing sequence databases. Unlike traditional database-dependent mass spectrometry approaches, De Novo techniques are not constrained by database completeness and allow comprehensive sequence determination—particularly beneficial for unknown antibodies or samples of complex origin.
A typical De Novo antibody sequencing workflow involves:
1. Sample preparation: enzymatic digestion of antibodies using proteases such as trypsin, chymotrypsin, or LysC;
2. Mass spectrometry analysis: acquisition of tandem MS/MS spectra using high-resolution mass spectrometers (e.g., Orbitrap or TOF);
3. Sequence interpretation: application of specialized De Novo algorithms (e.g., PEAKS, Novor) to derive peptide sequences;
4. Sequence assembly and reconstruction: reconstitution of complete light and heavy chain sequences by integrating antibody-specific structural features;
5. Functional validation: confirmation of sequence functionality through recombinant expression and analytical techniques such as ELISA or surface plasmon resonance (SPR).
Why Use De Novo Techniques for Antibody Sequencing?
1. Independence From mRNA Enables Application to Clinical Samples and Native Antibodies
In many real-world settings, such as antibodies isolated from serum or tissue fluids in clinical samples, relying on B-cell-derived mRNA is often impractical due to challenges in cell acquisition and low expression levels. De Novo techniques directly analyze the protein level, obviating the need for nucleic acid information, and thus offer unique advantages for studying naturally occurring antibodies.
2. Enhanced Accuracy and Coverage Through Advanced High-Resolution Mass Spectrometry
Ongoing advancements in mass spectrometry have enabled instruments to achieve sub-ppm mass accuracy and high sensitivity. When combined with multi-enzyme digestion strategies and optimized algorithms for overlapping peptides, De Novo sequencing can now achieve reported accuracies and coverage rates exceeding 95%, sufficient for most antibody engineering applications.
3. Capability to Identify Antibody Isoforms (e.g., Glycosylation, Mutations, Alternative Splicing)
Antibody diversity extends beyond amino acid sequences to include post-translational modifications (PTMs), such as glycosylation, oxidation, and deamidation, all of which significantly influence antibody function. Traditional nucleic acid-based sequencing methods cannot detect these modifications, whereas mass spectrometry provides direct, site-specific evidence of PTMs, enabling deeper functional insights.
Technical Challenges and Strategies in De Novo Antibody Sequencing
1. Difficulty in Resolving CDR Regions
The complementarity-determining regions (CDRs) of antibodies contain highly variable sequences that play a central role in antigen binding. These regions are often characterized by high sequence complexity and low enzymatic digestion efficiency, which together reduce their detectability in mass spectrometry analyses. MtoZ Biolabs addresses these challenges by employing strategies such as multi-enzyme digestion and isomer-focused analysis to enhance both the coverage and sequence reliability of CDR regions.
2. Challenges in Deconvoluting Homologous Antibody Mixtures
In samples containing multiple homologous antibodies—such as polyclonal antibodies or patient-derived antibodies before and after therapeutic intervention—traditional algorithms struggle to resolve highly similar sequences. MtoZ Biolabs combines deep sequencing coverage with intelligent algorithms and manual curation to enable accurate reconstruction of antibodies in highly complex biological matrices.
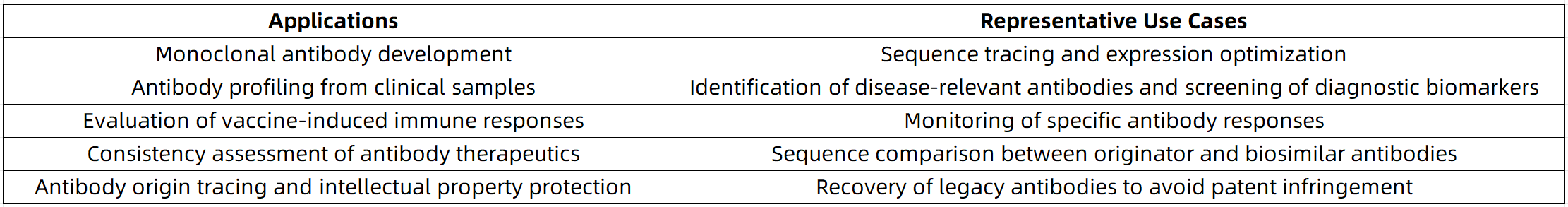
Application Scenarios: The Broad Value of De Novo Antibody Sequencing
MtoZ Biolabs: A High-Accuracy De Novo Antibody Sequencing Service Provider
At MtoZ Biolabs, we leverage the Orbitrap Fusion Lumos mass spectrometer along with optimized multi-enzyme digestion protocols and proprietary algorithms for sequence assembly and post-translational modification analysis. Our services include:
Our expert team brings extensive experience in mass spectrometry data interpretation and manually validates each project through rigorous sequence curation and structural modeling to ensure sequence reproducibility and functional relevance. As the fields of therapeutic antibody development and precision medicine continue to advance, high-quality antibody sequence information is increasingly recognized as a fundamental requirement. De Novo antibody sequencing has become an indispensable tool in both academic and industrial settings due to its high accuracy, versatility, and independence from reference sequences. MtoZ Biolabs remains committed to supporting researchers by unlocking the structural and functional insights of antibodies through advanced technical platforms and expert-driven services.
MtoZ Biolabs, an integrated chromatography and mass spectrometry (MS) services provider.
Related Services
How to order?







