HCP Antibody Effectiveness Analysis Service
1. Verification of HCP Antibody Coverage
There are primarily two methods for evaluating antibody coverage: 2D Western blot or Antibody Affinity Extraction (AAE).
(1) 2D Western Blot (2D-WB)
In the production and purification of therapeutic recombinant proteins expressed by cultured mammalian cells, contamination by HCPs is a potential issue. Every validation and quality control test of therapeutic proteins should include an assay to demonstrate HCP contamination levels. Typically, enzyme-linked immunosorbent assays (ELISAs) are used to detect residual mammalian HCPs in the purification process and the final drug product, regarded as the gold standard. HCPs include proteins with significant differences in molecular weight (MW), isoelectric point (pI), and hydrophobicity, whose complexity and heterogeneity pose challenges to detection and quantification. Two-dimensional gel electrophoresis (2-DE) is a powerful tool for analyzing complex protein spectra and is pivotal in biologics production. Gaining insights into HCP characteristics and compositions during purification is crucial; this knowledge aids in designing processes to minimize HCP presence in the final product. Moreover, integrating 2-DE with Western blotting supports ELISA development and quality control by estimating the detection capabilities of the employed polyclonal sera, allowing for comprehensive immunochemical analysis of HCPs.
This approach displays proteins in representative HCP samples as arrays of hundreds to thousands of distinct spots. Western blotting identifies which proteins are detectable by polyclonal antibodies. Therefore, a coverage fraction, representing the ratio of the number of proteins identified by the antibodies to the total number of detectable proteins in the sample, can be calculated. In simple terms, two gels can be run simultaneously, one for high-sensitivity fluorescence staining, and the other transferred to a PVDF membrane for staining and comparison with the other gel to verify transfer efficiency. By staining the blot membrane for total proteins and performing immunodetection using the anti-HCP antibody formulation under evaluation, one can compare the patterns of total proteins and immunodetected proteins on the same blot.
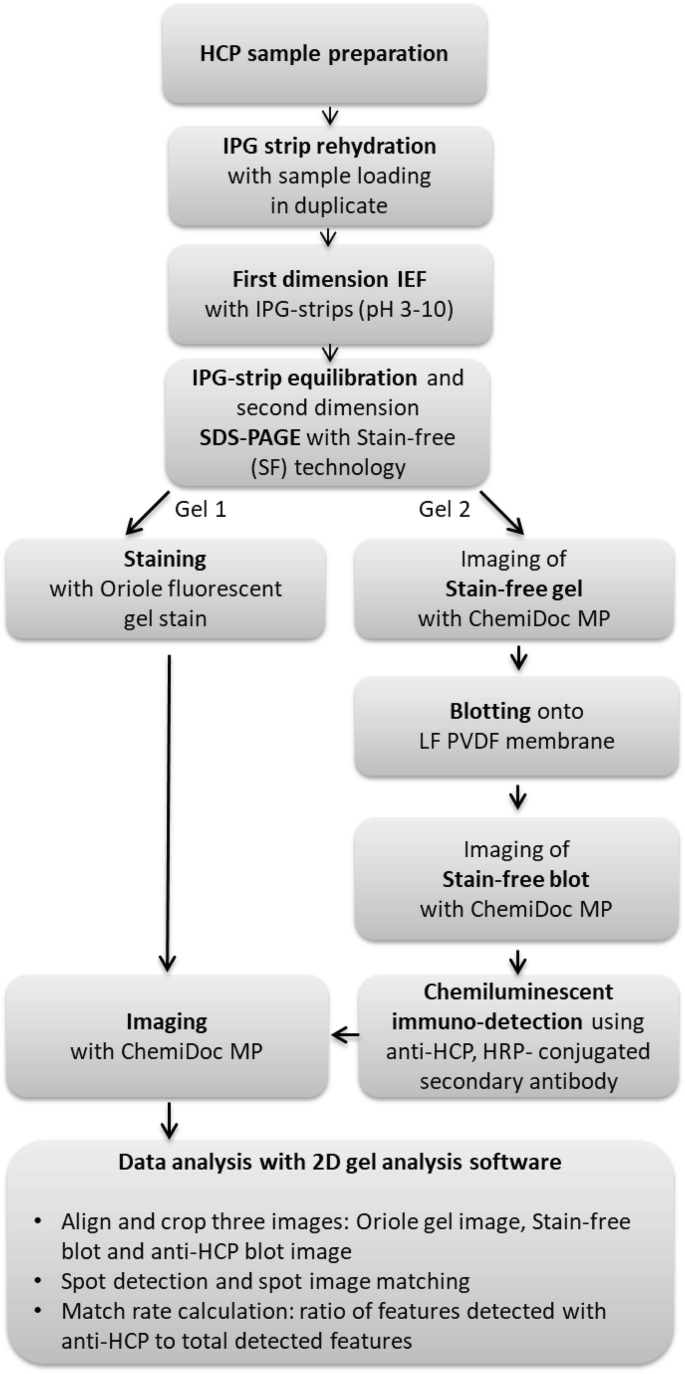
Figure 1. 2D-WB Procedure [3]
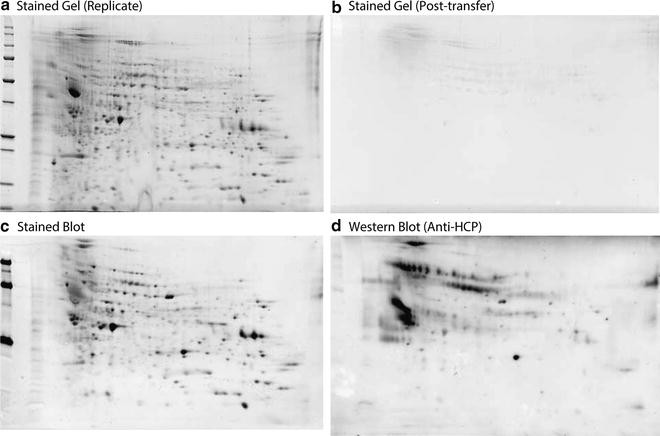
Figure 2. Common Results of 2D-WB [4]
(2) Immunoaffinity-Two-Dimensional Electrophoresis or Immunoaffinity-Mass Spectrometry Analysis
Mass spectrometry (MS) monitors thousands of potential HCPs, evaluating their relative or absolute quantities throughout the bioprocess and final bulk drug/product. Several studies have employed liquid chromatography (LC)-MS/MS for mapping HCPs in mAb bioprocesses, including 2D-LC-MS/MS and Isobaric Tags for Relative and Absolute Quantitation (iTRAQ) techniques. MS-based HCP quantification is also coupled with in silico predictions of specific HCP immunogenicity risks by identifying “foreign” epitopes in prevalent HCPs. The combination of ELISA immunocapture with LC-MS/MS has been used to assess the coverage of anti-HCP antibodies.
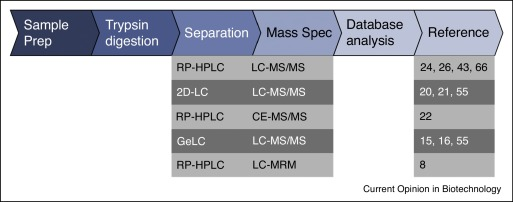
Figure 3. General Flowchart for HCP Analysis Using LC-MS [5]
Antibody Affinity Extraction (AAE) involves covalently fixing polyclonal antibodies to chromatography media. Native, undenatured HCP samples are passed through the column multiple times to bind with antibodies and then eluted until no HCPs remain bound. All HCP eluates are collected and concentrated to the original volume. The final samples undergo 2D SDS-PAGE separation and then compared to the initial untreated sample using silver staining, or Two-Dimensional Difference Gel Electrophoresis (2D-DIGE) with Cy3 and Cy5 labeling for pre- and post-extraction samples, respectively, to determine the antibody coverage of HCPs.
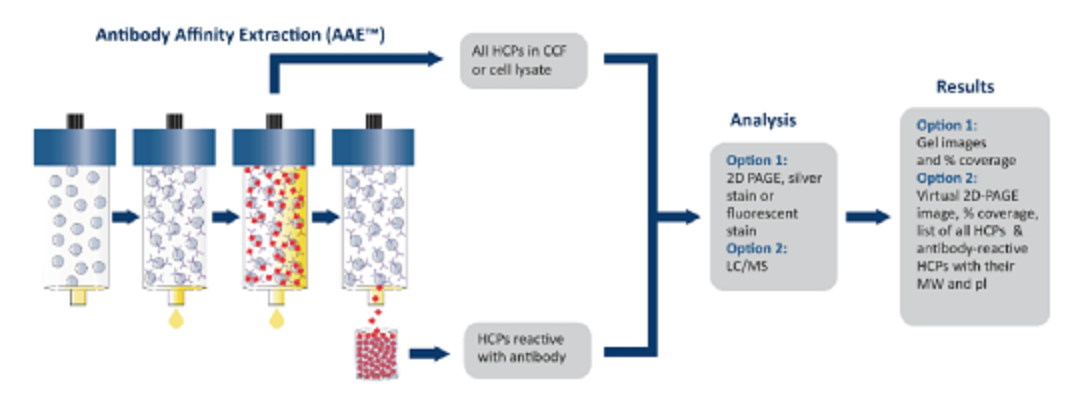
Figure 4. Immunoaffinity-Two-Dimensional Electrophoresis or Immunoaffinity-Mass Spectrometry (LC-MS/MS) Analysis Procedure
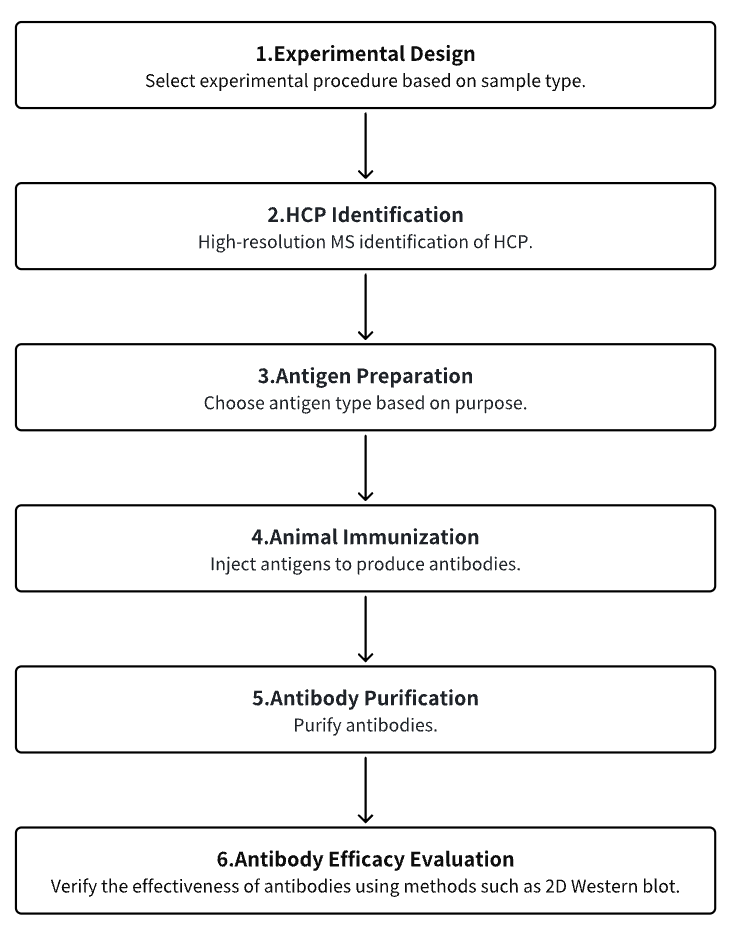
Analysis Workflow
1. Experimental Procedure Determination
2. HCP Identification
3. Host Cell Antigen Preparation
4. Animal Immunization
5. Antibody Purification
6. Antibody Efficacy Evaluation
Service Advantages
1. High Reliability Identification and Characterization of HCP
2. Multiple Antigen Preparation Platforms
3. Comprehensive and Strict Immunization Procedures
4. Effective Antibody Detection Systems and Extensive Detection Experience
Sample Results
1. Coverage Values Determined by 2D-WB: A Key Study in Characterizing Anti-HCP ELISA Reagents
HCPs are a major class of process-related impurities that must be extensively cleared through appropriate analytical methods to ensure product quality and reduce potential safety risks to patients. In this regard, the ELISA mainly relies on the quality of HCP reference standards (immunogens) and HCP-specific polyclonal antibodies and is considered the gold standard for HCP monitoring. To identify the quality of the antibodies used, 2D-WB is the preferred technique to determine antibody coverage, although it has practical limitations. Studies using various orthogonal methods, such as affinity-based MS and indirect ELISA, have revealed potential detection gaps (i.e., uncovered HCPs) in traditional 2D-WB, primarily attributable to two fundamental reasons: (i) insufficient quantity of proteins or antibodies to breach the detection limit; (ii) protein denaturation causing the loss of conformational epitopes, thereby hindering HCP-antibody recognition and leading to WB artifacts. Conversely, the lack of specific antibodies against certain HCPs (especially those of low MW), as previously suggested, appears to play a minimal role.
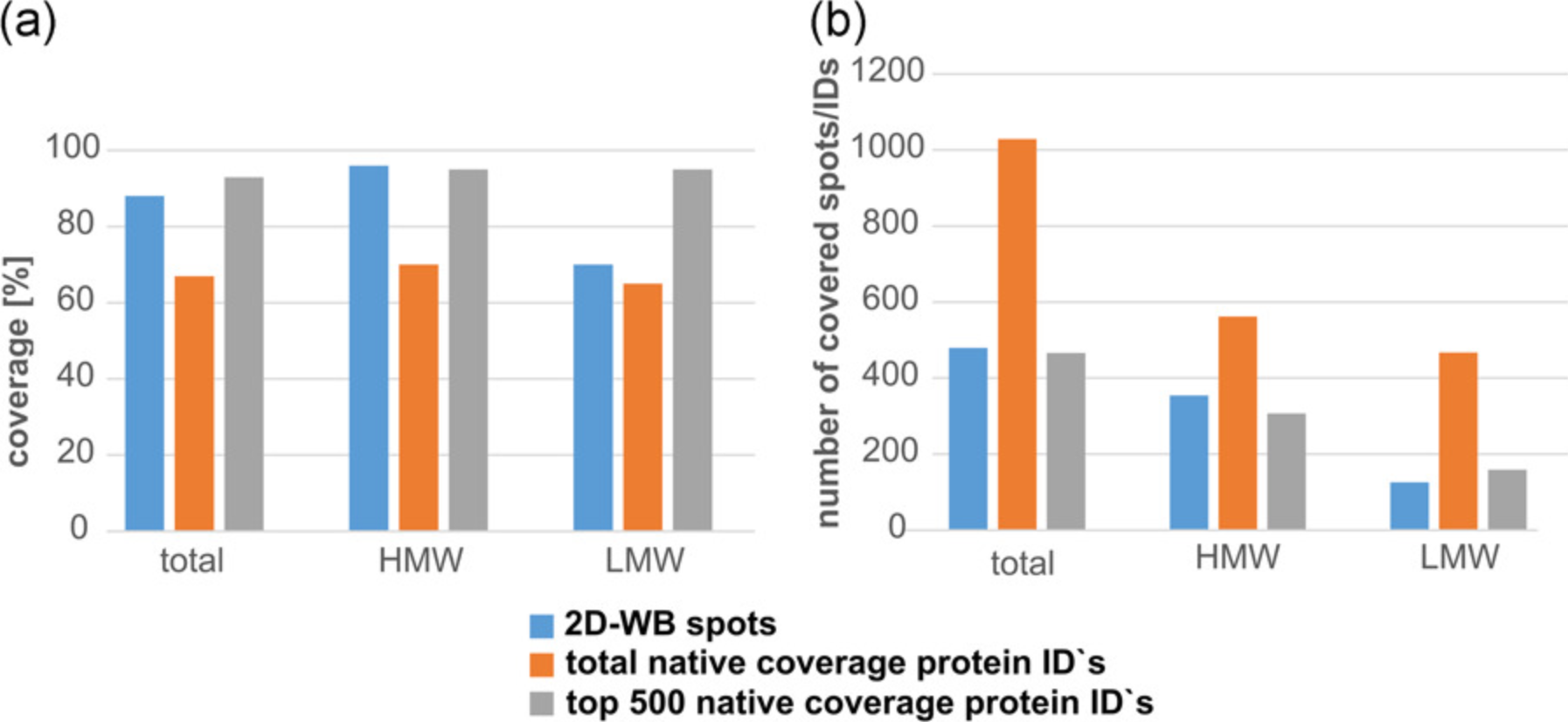
Figure 5. Coverage Rates by Different Methods [6]
2. A New Method for Assessing HCP ELISA Antibody Coverage-Combining ELISA-Based Immuno-Capture with MS
Monitoring HCPs is one of the most critical analytical requirements in recombinant biopharmaceutical production to ensure product purity and patient safety. The ELISA is the standard method for monitoring HCP clearance. It's vital to validate that the key reagents of ELISA (HCP antibodies) cover a wide range of HCPs potentially present in purified drugs. Current methods for assessing HCP antibody coverage are based on 2D-WB or combined 2-DE with immunoaffinity purification, but they have limitations. Research has introduced a new coverage method that combines ELISA-based immuno-capture with Liquid Chromatography-Tandem Mass Spectrometry (LC-MS/MS) protein identification: ELISA-MS. Using ELISA-MS, the coverage of three commercial anti-E. coli HCP antibodies for early process samples has been accurately determined, overcoming the limitations of current coverage analysis methods and utilizing the advantages of MS analysis. The results include lists of individual HCPs covered by each antibody. This new method is highly sensitive, reproducible, and strictly controls nonspecific binding through the addition of species-specific isotype control antibodies. Researchers believe that ELISA-MS will become an important supplement, even an alternative, to existing coverage methods. ELISA-MS will increase the likelihood of selecting the optimal HCP ELISA, thereby improving HCP monitoring and achieving the final HCP profile with the least risk. In summary, this benefits both the pharmaceutical industry and patient safety.
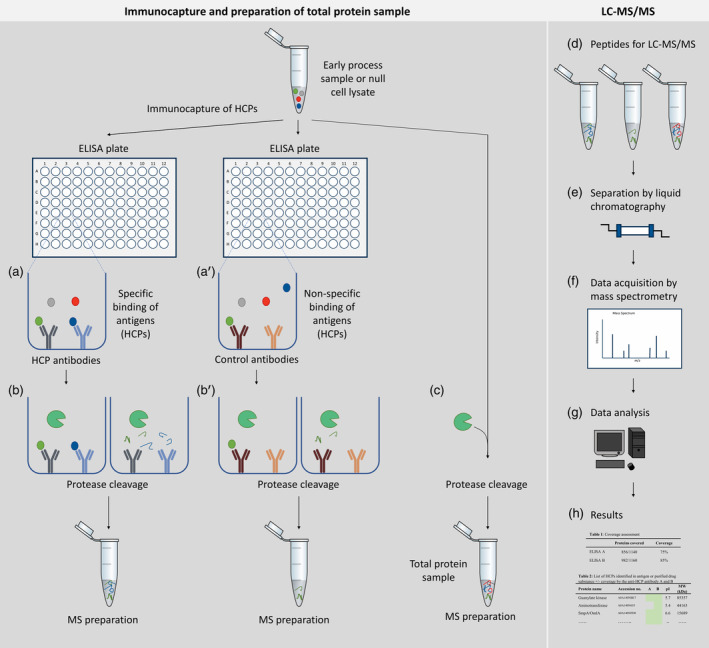
Figure 6. Protein Identification Through LC-MS/MS Based on ELISA Immuno-Capture [7]
3. Quantitative Analysis of L-Asparaginase and HCPs through SWATH LC-MS/MS for New and Improved Purification Steps in Erwinia-Asparaginase Production
L-Asparaginase is an indispensable part of treating children and adolescents with Acute Lymphoblastic Leukemia (ALL). E. chrysanthemi L-asparaginase was first developed in the early 1970s at Porton Down and is currently produced by Bolton Biopharmaceuticals Ltd. In the production process of E. chrysanthemi L-asparaginase, one of the early purification steps is using batch cation exchange carboxymethyl resin, and the replacement of this old technique with MS is currently under study to understand the impact of resin changes on the impurity profile. Research has developed a novel SWATH library targeting the E. chrysanthemi proteome and used it to assess the potential process change's impact on product yield, HCP characteristics, and clearance. Currently, ELISA is used as a quality control release test for quantitative HCP in the Drug Substance (DS) phase, but these early extract samples are too crude for interference-free analysis by ELISA. Given the ELISA method's limitations in evaluating new resin choices, SWATH LC-MS/MS analysis has proven key in selecting resins for further amplification and implementation. Data indicates that the concentration of L-asparaginase in the new process steps is 2.28 times that of traditional process samples. The new steps using modern ion exchangers are at least equivalent to traditional resin steps in clearing 78.2% of HCPs (528 of 675 proteins), and in some cases, even superior.
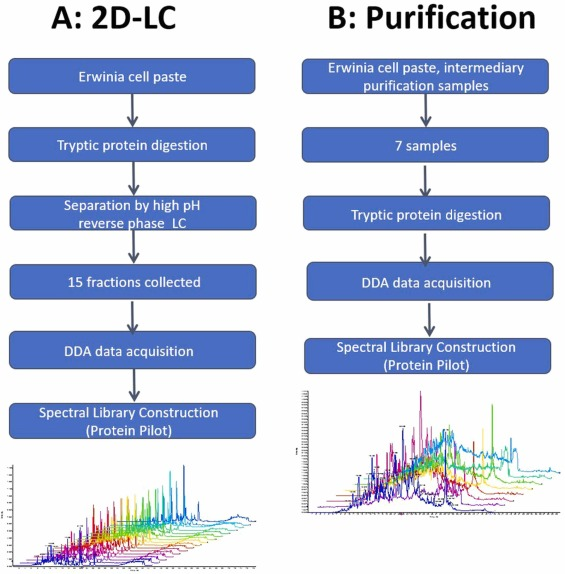
Figure 7. Workflow for Creating SWATH Spectral Libraries Using DDA-MS Analysis [8]
4. Assessing ELISA Reagent Coverage via Affinity Purification-Mass Spectrometry
It's imperative to sufficiently remove host cell proteins (HCPs) from recombinant therapeutic drugs during downstream processing to guarantee patient safety, uphold product quality, and meet regulatory standards. The monitoring of HCP clearance often employs ELISA using polyclonal agents. Although MS is adept at identifying and tracking specific HCP process contaminants, ELISA is favored for its simplicity and high throughput. Integrating protein-focused MS characterizations with ELISA, or ensuring ELISA cover all specific process-related HCP impurities that could pose safety or efficacy risks, presents inherent difficulties. Research has introduced the effective determination of ELISA reagent coverage through proteome analysis following the affinity purification using polyclonal anti-HCP reagents (AP-MS). The resulting HCP identification can be compared with actual downstream process impurities of a specific process, thereby allowing for a highly focused evaluation of the ELISA reagent's suitability. An evaluation of the coverage of polyclonal antibodies against HCPs was conducted on downstream HCP impurities found in therapeutic monoclonal antibodies post Protein A purification, demonstrating the practicality of this method.
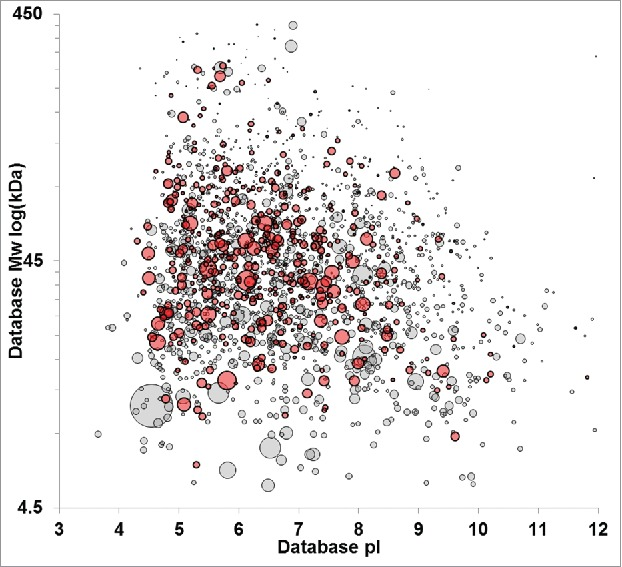
Figure 8. 2D-PAGE Comparison of HCP with/without Defined Immunoreactivity [9]
5. Universal Affinity-Based MS for In-Depth ELISA Reagent Coverage Evaluation
ELISA stands as the preferred quantification method for HCPs in biopharmaceutical processing. Besides 2D-WB, affinity purification-based liquid chromatography-tandem mass spectrometry (AP-MS) is increasingly used for assessing ELISA antibody coverage. However, all these methods face the issue of nonspecific binding between antibodies and the matrix, which involves the application of arbitrarily defined thresholds during the data evaluation process. Addressing this challenge, an optimized AP-MS method has been developed, employing cleavable linkers attached to ELISA antibodies to isolate HCPs engaged in specific interactions. By comparing the method variability and the number of false positive or false negative hits between two methods, we can demonstrate that the optimized AP-MS method exhibits strong repeatability and superiority in identifying gaps in antibody detection, whereas the previously described strategies are prone to overestimating or underestimating coverage. This approach, focusing exclusively on antibody-related HCPs, has proven valuable for precise hitchhiker analysis. Collectively, this innovative method is affirmed as an effective tool for accurately assessing ELISA antibody coverage.
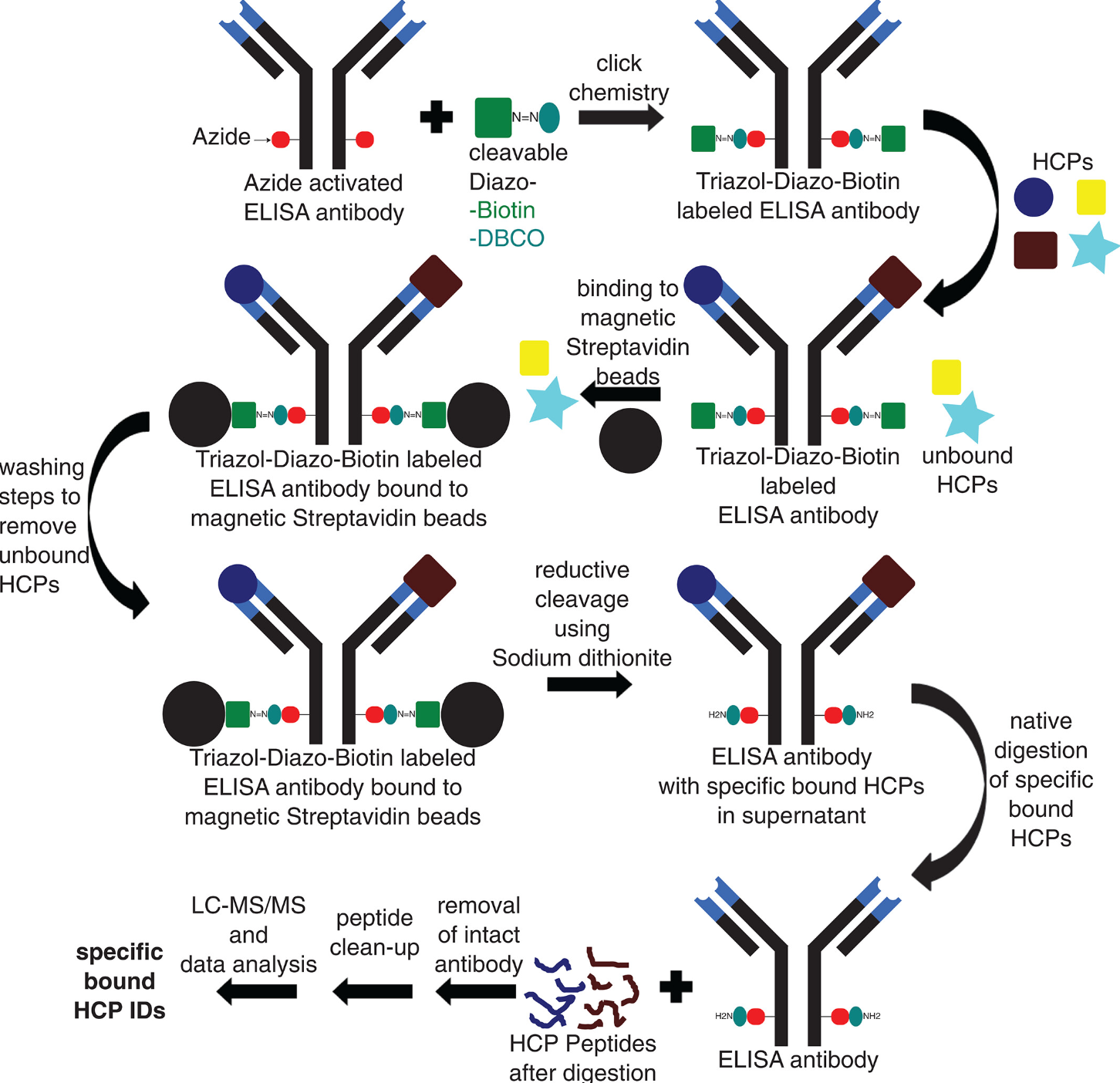
Figure 9. Optimized AP-MS Method Workflow [10]
Sample Submission Requirements
1. Minimize Impurity Contamination
Services at MtoZ Biolabs
1. Complete Experimental Procedure
2. Relevant Instrument Parameters
3. Original Experimental Data
4. Data Analysis Submission
References
[1] Tuameh A, Harding SE, Darton NJ. Methods for addressing host cell protein impurities in biopharmaceutical product development. Biotechnol J. 2023 Mar;18(3):e2200115. doi: 10.1002/biot.202200115. Epub 2022 Dec 13. PMID: 36427352.
[2] Haltaufderhyde K, Roberts BJ, Khan S, Terry F, Boyle CM, McAllister M, Martin W, Rosenberg A, De Groot AS. Immunoinformatic Risk Assessment of Host Cell Proteins During Process Development for Biologic Therapeutics. AAPS J. 2023 Sep 11;25(5):87. doi: 10.1208/s12248-023-00852-z. Erratum in: AAPS J. 2023 Dec 19;26(1):6. PMID: 37697150.
[3] Posch A, Kollmann F, Berkelman T, Dreskin E. Sample Preparation of Secreted Mammalian Host Cell Proteins and Their Characterization by Two-Dimensional Electrophoresis and Western Blotting. Methods Mol Biol. 2021;2261:507-524. doi: 10.1007/978-1-0716-1186-9_32. PMID: 33421011.
[4] Berkelman T, Harbers A, Bandhakavi S. 2-D Western blotting for evaluation of antibodies developed for detection of host cell protein. Methods Mol Biol. 2015;1295:393-414. doi: 10.1007/978-1-4939-2550-6_28. PMID: 25820736.
[5] Bracewell DG, Smith V, Delahaye M, Smales CM. Analytics of host cell proteins (HCPs): lessons from biopharmaceutical mAb analysis for Gene therapy products. Curr Opin Biotechnol. 2021 Oct;71:98-104. doi: 10.1016/j.copbio.2021.06.026. Epub 2021 Jul 24. PMID: 34311150.
[6] Seisenberger C, Graf T, Haindl M, Wegele H, Wiedmann M, Wohlrab S. Questioning coverage values determined by 2D western blots: A critical study on the characterization of anti-HCP ELISA reagents. Biotechnol Bioeng. 2021 Mar;118(3):1116-1126. doi: 10.1002/bit.27635. Epub 2020 Dec 4. PMID: 33241851.
[7] Pilely K, Nielsen SB, Draborg A, Henriksen ML, Hansen SWK, Skriver L, Mørtz E, Lund RR. A novel approach to evaluate ELISA antibody coverage of host cell proteins-combining ELISA-based immunocapture and mass spectrometry. Biotechnol Prog. 2020 Jul;36(4):e2983. doi: 10.1002/btpr.2983. Epub 2020 Mar 9. PMID: 32087048; PMCID: PMC7507178.
[8] Modi T, Regufe da Mota S, Gervais D. l-Asparaginase and HCP quantification by SWATH LC-MS/MS for new and improved purification step in Erwinia chrysanthemil-asparaginase manufacture. J Pharm Biomed Anal. 2022 Feb 5;209:114537. doi: 10.1016/j.jpba.2021.114537. Epub 2021 Dec 14. PMID: 34929569.
[9] Henry SM, Sutlief E, Salas-Solano O, Valliere-Douglass J. ELISA reagent coverage evaluation by affinity purification tandem mass spectrometry. MAbs. 2017 Oct;9(7):1065-1075. doi: 10.1080/19420862.2017.1349586. Epub 2017 Jul 14. PMID: 28708446; PMCID: PMC5627587.
[10] Seisenberger C, Graf T, Haindl M, Wegele H, Wiedmann M, Wohlrab S. Toward optimal clearance: A universal affinity-based mass spectrometry approach for comprehensive ELISA reagent coverage evaluation and HCP hitchhiker analysis. Biotechnol Prog. 2022 May;38(3):e3244. doi: 10.1002/btpr.3244. Epub 2022 Mar 11. PMID: 35150475.
MtoZ Biolabs, an integrated chromatography and mass spectrometry (MS) services provider.
Related Services
How to order?







