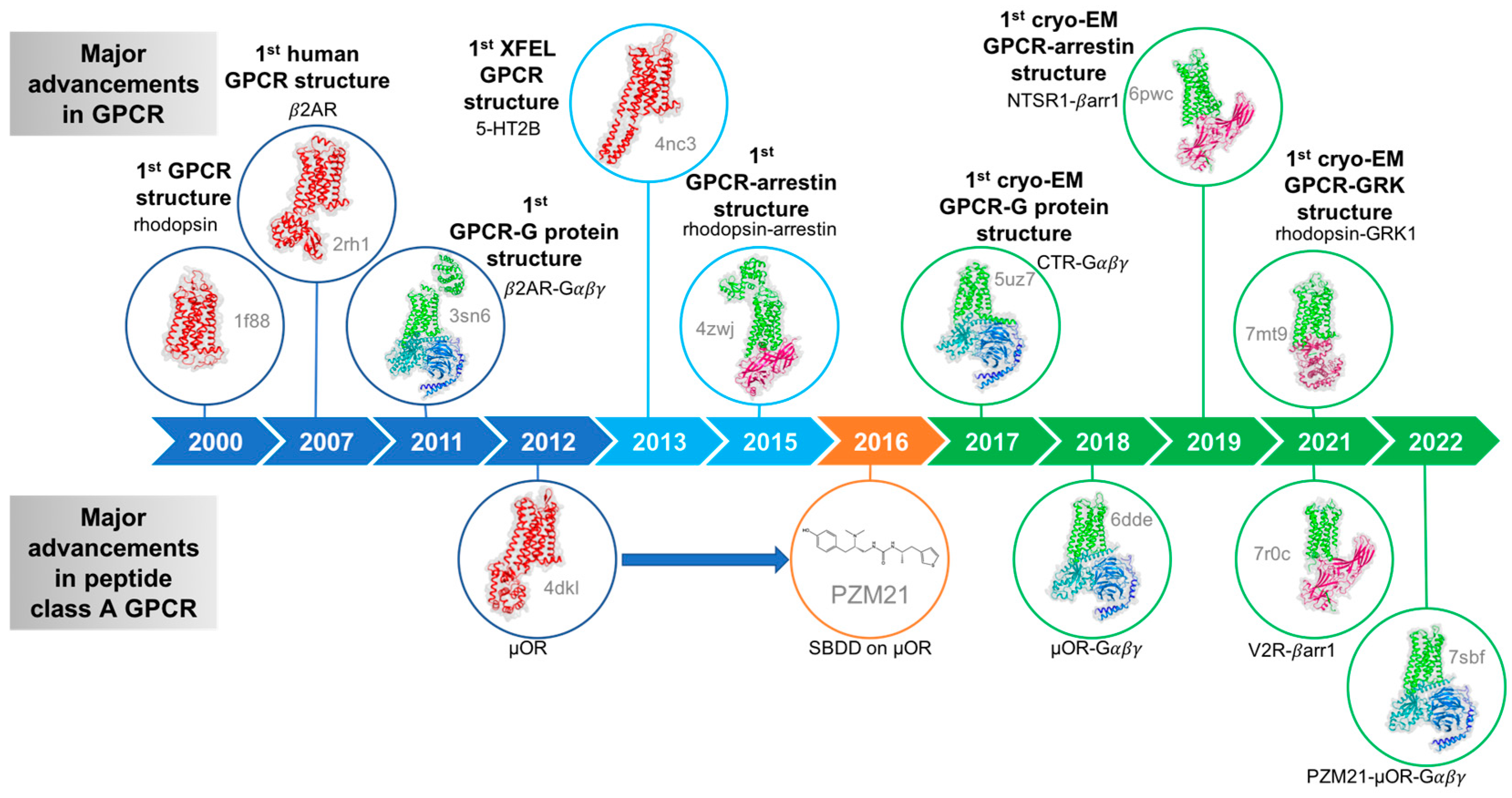
GPCRs Structure Characterization Service
GPCRs Structure Characterization Service refers to the use of cryo-electron microscopy (Cryo-EM) to perform high-resolution three-dimensional structural analysis of G protein-coupled receptors (GPCRs), including their ligand-bound complexes and signaling assemblies (such as GPCR-G protein-ligand ternary complexes), without the need for crystallization. This service enables the precise elucidation of receptor conformations, ligand-binding sites, activation mechanisms, and signal transduction interfaces.
GPCRs constitute the largest superfamily of membrane proteins, regulating a wide range of physiological processes in the human body, including sensory perception, immune responses, metabolism, and neural signaling. Due to their critical roles in disease pathogenesis, approximately one-third of all marketed drugs target GPCRs either directly or indirectly. However, GPCRs exhibit high flexibility, multiple conformational states, dynamic signaling behavior, low expression levels, and membrane dependence, making them extremely challenging for traditional structural biology methods such as X-ray crystallography and NMR to resolve efficiently, particularly when analyzing their full-length conformations or their dynamic structures during ligand binding and signal activation.
Fouillen A. et al. Membranes. 2023.
Cryo-EM is a technique that captures images of frozen biological samples under ultra-low temperature conditions using an electron beam, and reconstructs their three-dimensional molecular structures through single-particle analysis. Cryo-EM offers unique advantages, including no requirement for crystallization, near-native state preservation, and adaptability to dynamic and heterogeneous molecular systems, making it particularly suitable for studying membrane proteins, large macromolecular complexes, and flexible domains. For complex transmembrane protein systems such as GPCRs, Cryo-EM—combined with stabilization strategies—has become the mainstream method for resolving native conformations, ligand-binding modes, and activation states.
Leveraging an advanced cryo-EM platform, MtoZ Biolabs offers the GPCRs Structure Characterization Service capable of resolving both static conformations (Inactive/Active states) and dynamic intermediate states (partially activated or transitional conformations). This service is particularly suitable for analyzing native GPCRs, low-abundance receptors, dynamic activation conformations, and multi-conformational systems that are difficult to resolve using traditional methods, providing critical structural insights for drug discovery, mechanistic studies, and structure-based optimization.
Analysis Workflow
GPCRs Structure Characterization Service provided by MtoZ Biolabs follows these key steps:
1. Sample Preparation and Complex Stabilization
Express and purify GPCRs; stabilize complexes by incorporating nanobodies, mini-G proteins, or specific receptor mutants.
2. Cryo-Grid Preparation and Screening
Prepare cryo-EM grids with optimized ice thickness and screen initial grids to assess particle distribution and orientation features.
3. High-Throughput Cryo-EM Data Collection
Acquire large datasets of high-quality images using cryo-EM, capturing the in situ state of the receptor complexes.
4. Image Processing and 3D Reconstruction
Perform particle picking, 2D/3D classification, and 3D reconstruction to generate high-resolution density maps from selected particles.
5. Model Building and Structural Analysis
Construct atomic models of the GPCR-ligand-G protein complexes, annotate binding interfaces, and analyze activation mechanisms.
6. Data Delivery and Functional Interpretation
Deliver standardized datasets and structural reports suitable for scientific publication, drug design, and mechanistic research applications.
Applications
Structure-Based Drug Design (SBDD)
Accurately resolve binding modes of small molecules, peptides, and antibodies to guide lead optimization and Hit-to-Lead transitions.
Receptor Activation and Signal Transduction Mechanism Studies
Elucidate the conformational transitions of GPCR activation, G protein coupling modes, and downstream signaling mechanisms.
Multi-Ligand and Multi-Conformation Analysis
Compare the binding and activation differences among agonists, antagonists, and partial agonists targeting the same GPCR.
Viral GPCR (vGPCR) and Pathological Receptor Research
Support the structural characterization of pathogen-related GPCR targets (e.g., CMV GPCRs) for antiviral drug development.
FAQ
Q. Must GPCRs be complexed with ligands or G proteins to perform Cryo-EM structural analysis?
It is strongly recommended. Apo (ligand-free) GPCRs are highly dynamic and heterogeneous, making it difficult to achieve high-resolution reconstructions. Ligand binding and G protein coupling help lock the receptor into specific functional states, greatly improving particle uniformity, orientation distribution, and final map quality.
Q. Can Cryo-EM capture subtle conformational differences induced by different ligands (agonists, antagonists, biased ligands)?
Yes. High-resolution Cryo-EM (better than 3.5 Å) combined with 3D classification and local refinement can accurately detect fine conformational shifts, such as microswitch displacements or pocket opening/closing, providing insight into activation, antagonism, and biased signaling mechanisms.
Q. If GPCR sample expression is low or complex stability is moderate, can Cryo-EM analysis still be performed?
It is possible, but success depends on sample purity, homogeneity, and particle behavior. We offer stabilization strategies (e.g., incorporation of nanobodies, mini-G proteins, or stabilizing mutations) and provide pre-screening services to maximize the feasibility of successful structural analysis.
Case Study
This study elucidated the stepwise allosteric activation mechanism of the class C G protein-coupled receptor dimer mGlu5. By capturing the three-dimensional conformation of mGlu5 in different activation states through Cryo-EM, the study found that agonist binding induced compression of the extracellular domains (ectodomains), and the signal was transmitted to the seven transmembrane regions (7TM domains) in the membrane through the semi-rigid cysteine-rich domain (CRD), forming an asymmetric TM6-TM6 interface, while promoting the opening of the ICL2 region to form a pseudo-cavity that is conducive to G protein binding. This research system revealed the structural basis of the step-by-step allosteric activation of mGlu5 dimers on a millisecond scale, providing important clues for understanding the activation process of class C GPCRs and developing new regulatory drugs.
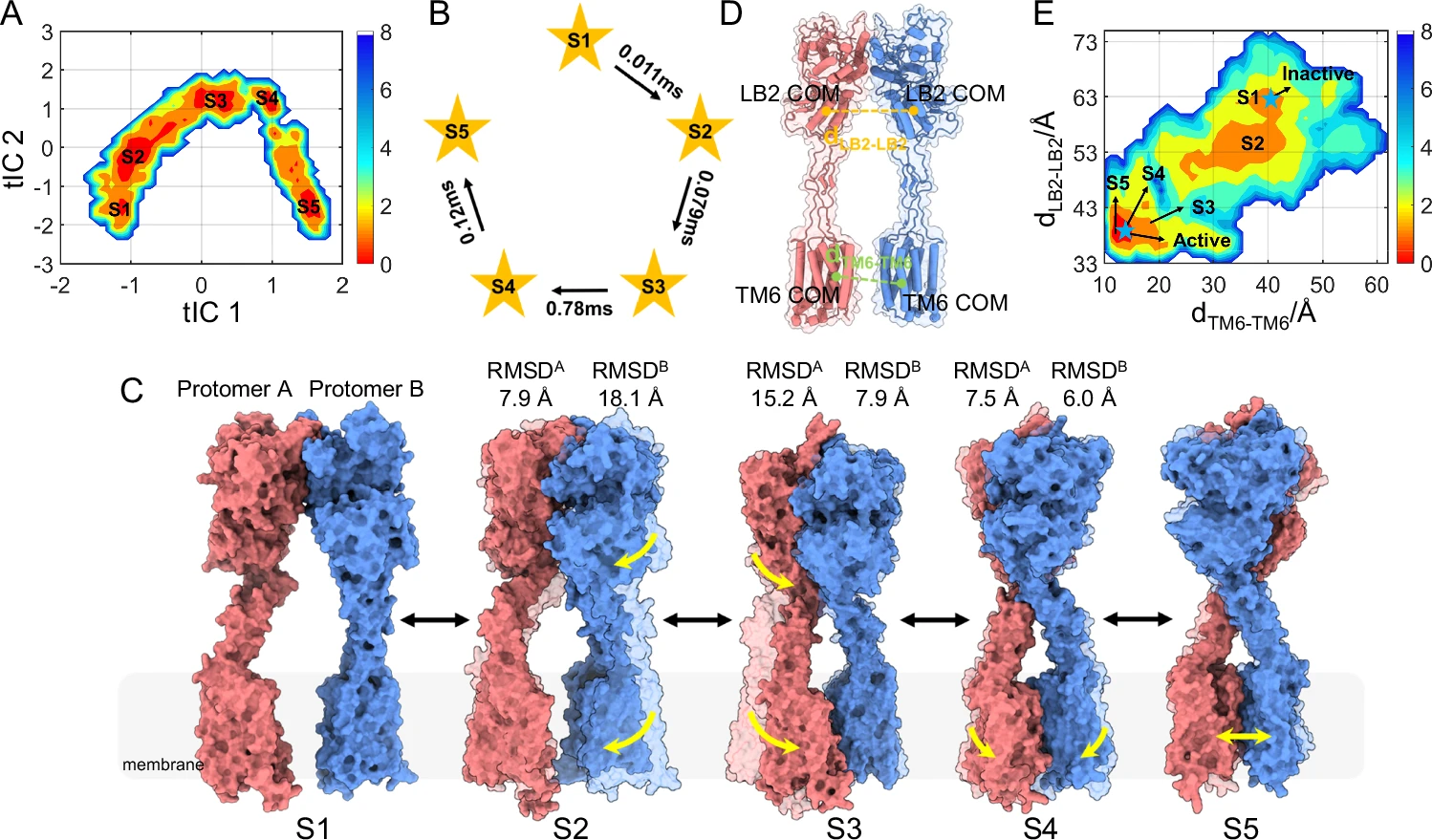
Li M. et al. Nat Commun. 2024.
How to order?







