Extracellular Vesicle Manufacturing Service
Extracellular vesicle manufacturing refers to a specialized service that produces large quantities of well-defined and quality-stable extracellular vesicles (EVs) through standardized and scalable methods to meet research and clinical demands. Extracellular vesicles are nanoscale vesicles secreted by cells, carrying various biomolecules such as proteins, RNA, and lipids. They play critical roles in intercellular communication, material transport, and signal regulation, and are characterized by excellent biocompatibility and low immunogenicity. Through optimized cell culture systems and efficient isolation and purification techniques, this service enables the production of high-purity and high-activity vesicles, as well as customizable modifications or drug loading of vesicles.
Extracellular vesicle manufacturing service is widely applied in fields such as cell therapy, gene therapy, drug delivery, vaccine development, and immune regulation, with particular significance in developing innovative therapies for cancer, immune diseases, and neurodegenerative disorders. In addition, this service provides standardized sources of vesicles for fundamental research, functional validation, and mechanistic studies, thereby facilitating the translation of extracellular vesicle research from basic science to clinical applications.
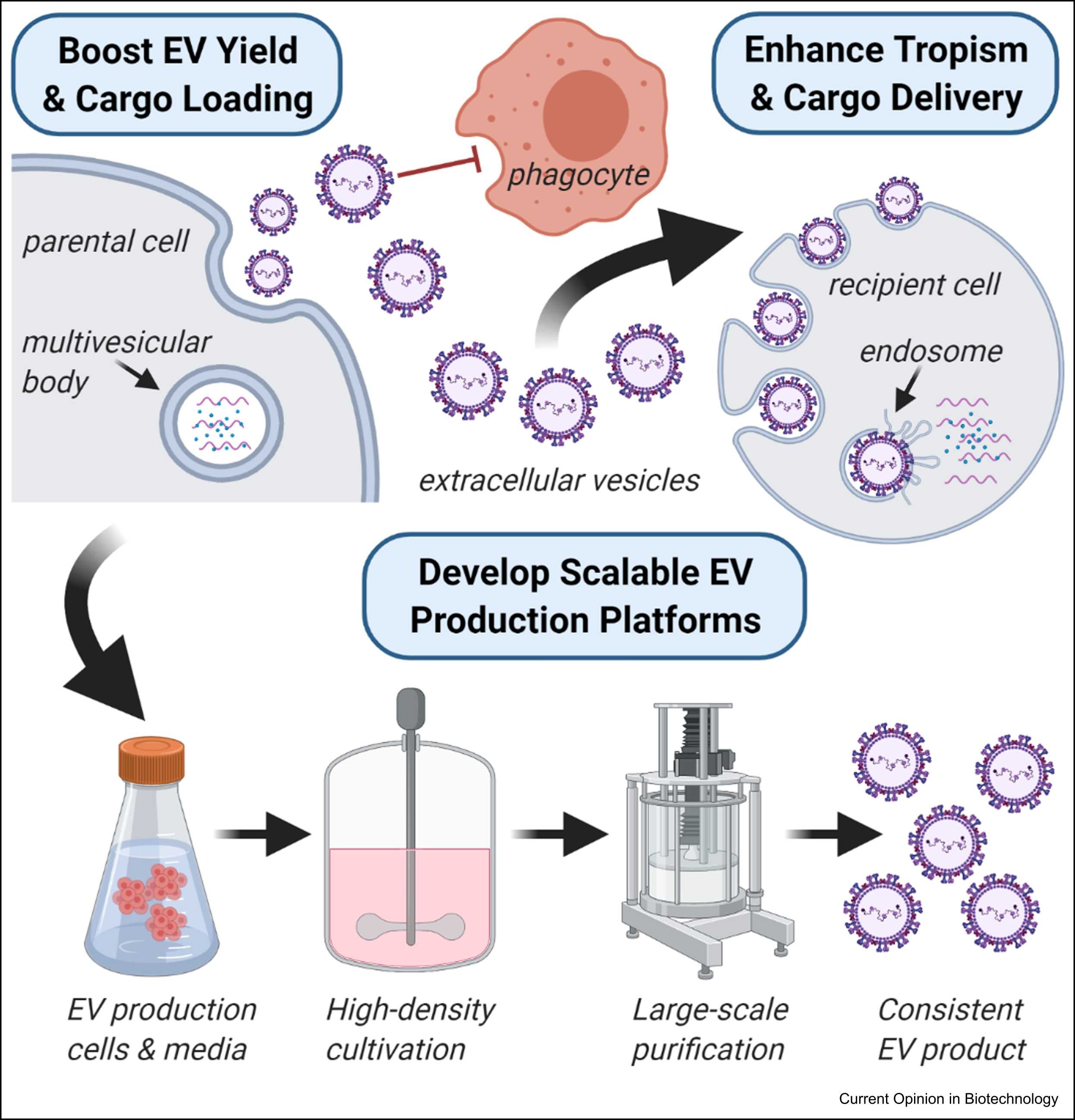
Estes, S. et al. Current Opinion in Biotechnology, 2022.
Figure 1. Challenges and Opportunities for Large-Scale EV Biomanufacturing.
Services at MtoZ Biolabs
Relying on advanced cell culture and isolation-purification platforms, MtoZ Biolabs offers extracellular vesicle manufacturing service to meet high-quality, standardized production demands. Through optimized cell culture systems, high-efficiency ultracentrifugation, density gradient separation techniques, and verification by transmission electron microscopy (TEM), we ensure the production of high-purity, high-activity, and well-defined extracellular vesicles. Additionally, we provide comprehensive vesicle characterization and analysis services, including particle size distribution, concentration measurement, and surface marker profiling, to guarantee vesicle quality and consistency, fulfilling the diverse needs of both basic research and clinical applications.
Service Advantages
1. High Purity and High Quality
Through standardized cell culture and multi-step purification processes, we ensure the acquisition of high-purity and highly intact extracellular vesicles, meeting the demands of both research and applied applications.
2. One-Time-Charge
Our pricing is transparent, no hidden fees or additional costs.
3. Multiple Source Support
Extracellular vesicles can be manufactured from various cell types according to customer needs, meeting diverse research and therapeutic applications.
4. Customized Services
MtoZ Biolabs offers engineering customization services, including surface modification and functional molecule loading, to produce extracellular vesicles tailored to specific research goals.
Applications
1. Drug Delivery Systems
Extracellular vesicle manufacturing service can produce engineered extracellular vesicles for delivering small-molecule drugs, RNA, proteins, and more, enhancing drug targeting and bioavailability.
2. Cell Therapy and Immunotherapy
As cell-free therapeutic carriers, extracellular vesicles are applied in tumor immunotherapy, autoimmune disease treatment, and tissue regeneration, serving as alternatives or complements to traditional cell therapies.
3. Disease Models and Mechanism Studies
Extracellular vesicle manufacturing service can provide well-defined and high-quality vesicles for basic research, enabling the study of extracellular vesicles' roles in disease pathogenesis and cell communication.
4. Biomarker Development
By producing vesicles from specific sources, this service supports the discovery and validation of disease-associated protein, RNA, and lipid biomarkers, advancing precision medicine technologies such as liquid biopsy.
Case Study
1. Manufacture of Extracellular Vesicles Derived from Mesenchymal Stromal Cells
This study aimed to optimize and evaluate the manufacturing methods of extracellular vesicles (EVs) derived from mesenchymal stromal cells (MSCs). The study focused on MSC-derived EVs and explored various isolation and purification techniques to improve vesicle yield and purity. Methods included commonly used techniques such as ultracentrifugation, density gradient centrifugation, and immunoaffinity capture, along with vesicle characterization by nanoparticle tracking analysis (NTA) and transmission electron microscopy (TEM). Results demonstrated that MSC-derived EVs obtained through optimized isolation processes exhibited high purity and quality, carrying diverse biomolecules such as proteins, RNAs, and lipids, and displaying robust biological functionality. The study further found that RNAs and proteins within the EVs played significant roles in immune regulation and tissue repair under various biological conditions. The conclusion highlighted that MSC-derived EVs hold great potential in cell therapy, drug delivery, and immunotherapy, and that the optimized manufacturing methods provide a solid foundation for their clinical application.
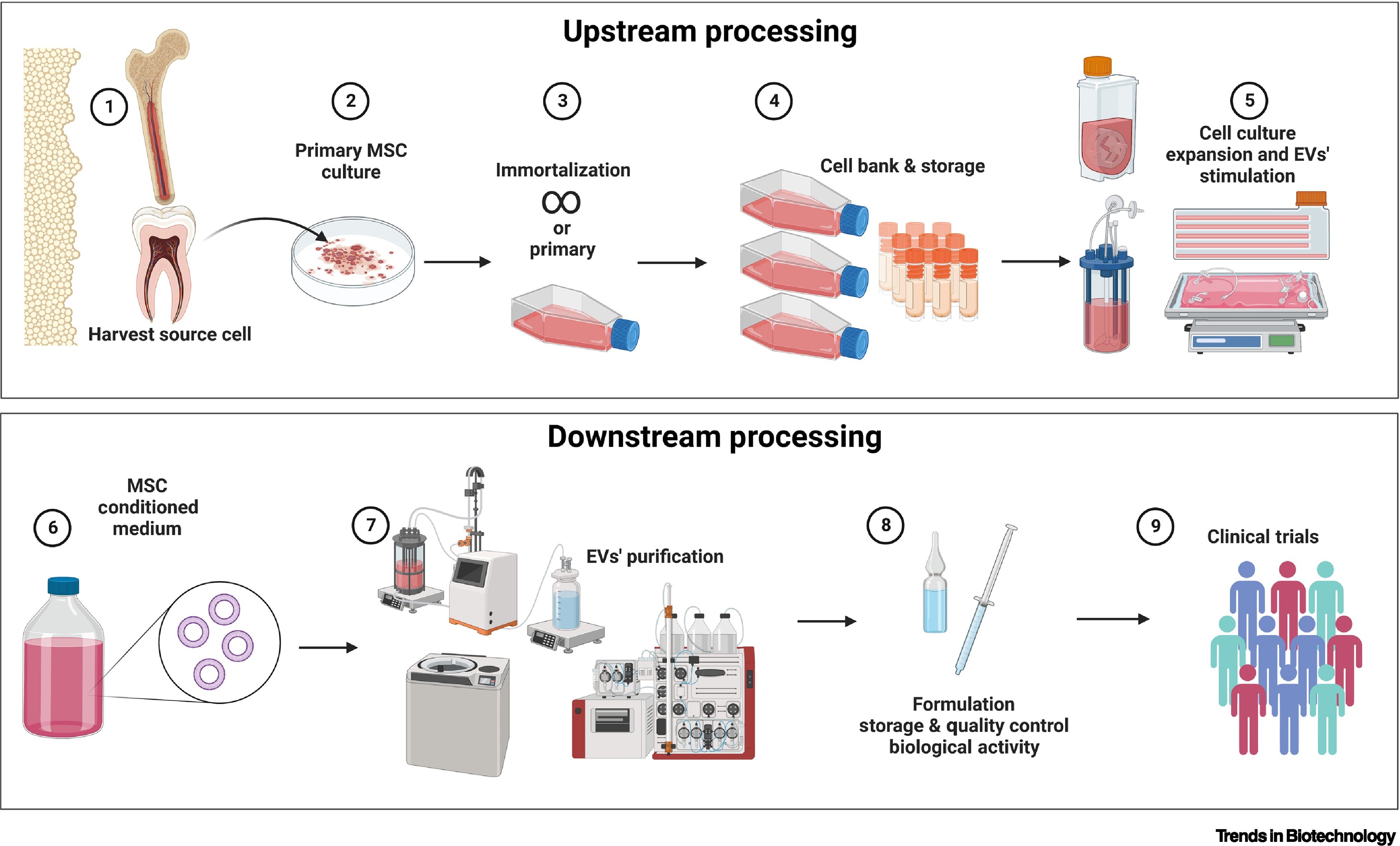
Lins, P M P. et al. Trends in Biotechnology, 2023.
Figure 2. Manufacturing Scheme of MSC-EVs as Next-Generation Therapeutics.
MtoZ Biolabs, an integrated Chromatography and Mass Spectrometry (MS) Services Provider, provides advanced proteomics, metabolomics, and biopharmaceutical analysis services to researchers in biochemistry, biotechnology, and biopharmaceutical fields. Our ultimate aim is to provide more rapid, high-throughput, and cost-effective analysis, with exceptional data quality and minimal sample consumption. Free project evaluation, welcome to learn more details!
How to order?







