Exosome Manufacturing Service
Exosome manufacturing service refers to the standardized, scalable production of exosomes under controlled conditions for research, diagnostic, or therapeutic applications. To meet the increasing demand for high-quality and application-specific exosomes, MtoZ Biolabs offers a comprehensive Exosome Manufacturing Service. Our Exosome Manufacturing Service supports custom exosome production from a variety of biological sources. We provide end-to-end solutions encompassing upstream production, purification, characterization, and application support.
Service at MtoZ Biolabs
✔️Types of Exosomes We Can Manufacture
1. Animal Cell-derived Exosomes
Sourced from animal cells such as HEK293, mesenchymal stem cells (MSCs), immune cells, or tumor cells, these exosomes are widely used in drug delivery, immunomodulation, tissue regeneration, and cancer research. They exhibit cell-specific cargo profiles and membrane markers that make them suitable for targeted therapy and functional studies.
2. Plant-derived Exosomes
Extracted from plant sources like ginger, grapes, and citrus, these vesicle-like structures possess natural biocompatibility and low immunogenicity. Plant-derived exosomes are gaining traction in oral drug delivery, gut–immune regulation, and anti-inflammatory applications, offering a safe and sustainable alternative to animal-derived exosomes.
3. Microorganism-derived Exosomes
Produced by bacteria or fungi, these exosome particles can carry microbial enzymes, lipoproteins, and nucleic acids. They are actively explored for vaccine development, microbiome studies, and host-pathogen interaction research due to their strong immunostimulatory potential.
4. Body Fluid-derived Exosomes
Isolated from human or animal body fluids such as plasma, serum, urine, saliva, or milk, these exosomes reflect the physiological or pathological state of the donor. They are extensively used in biomarker discovery, disease diagnostics, and non-invasive monitoring, particularly in oncology, neurology, and metabolic disease research.
✔️Exosomes Production Process
To ensure exosome quality, scalability, and downstream functionality, MtoZ Biolabs follows a standardized, four-stage manufacturing process:
1. Donor Cell Preparation
We establish and expand validated donor cell—including animal cells, plant cells, microbial strains, or custom cell types—under EV-compatible and serum-free conditions. Cell health, viability, and passage control are rigorously monitored to ensure consistent exosome output.
2. Exosome Manufacturing
Exosomes are isolated from conditioned media using a combination of ultracentrifugation, tangential flow filtration (TFF), and size exclusion chromatography (SEC), selected based on sample type and purity requirements. These methods enable efficient removal of debris and soluble contaminants while preserving vesicle integrity. In addition, we offer exosome engineering services such as cargo loading (e.g., RNA, proteins, small molecules) and surface modification (e.g., ligand or peptide display) to support advanced functional applications.
3. Exosome Characterization and Analysis
We provide comprehensive exosome characterization, including particle size and concentration analysis (NTA), morphological evaluation (TEM), and surface marker profiling. To support in-depth functional studies, we also offer multi-omics analysis, such as proteomics, transcriptomics, and lipidomics, enabling detailed profiling of exosomal cargo.
4. Exosome Lyophilization
For long-term storage and transport, exosomes can be lyophilized under controlled cryoprotectant conditions. This preserves vesicle integrity and bioactivity, facilitating downstream application without compromising quality.
✔️Evaluation Services for Manufactured Exosome
To ensure reproducibility, safety, and functional reliability, we provide comprehensive exosome manufacturing assessment, including:
1. Stability Evaluation: Examine exosome integrity and cargo preservation under storage and handling conditions.
2. Purity Evaluation: Detect and eliminate contaminants such as free proteins, debris, or non-exosomal vesicles.
3. Reproducibility Evaluation: Ensure batch-to-batch consistency in size, yield, and marker expression.
4. Production Efficiency Evaluation: Measure yield per input volume or cell biomass to optimize production cost-effectiveness.
5. Specificity Evaluation: Assess targeting properties based on ligand display or surface marker expression.
6. Safety Evaluation: Endotoxin testing, sterility, and potential immunogenicity assessment for in vivo use.
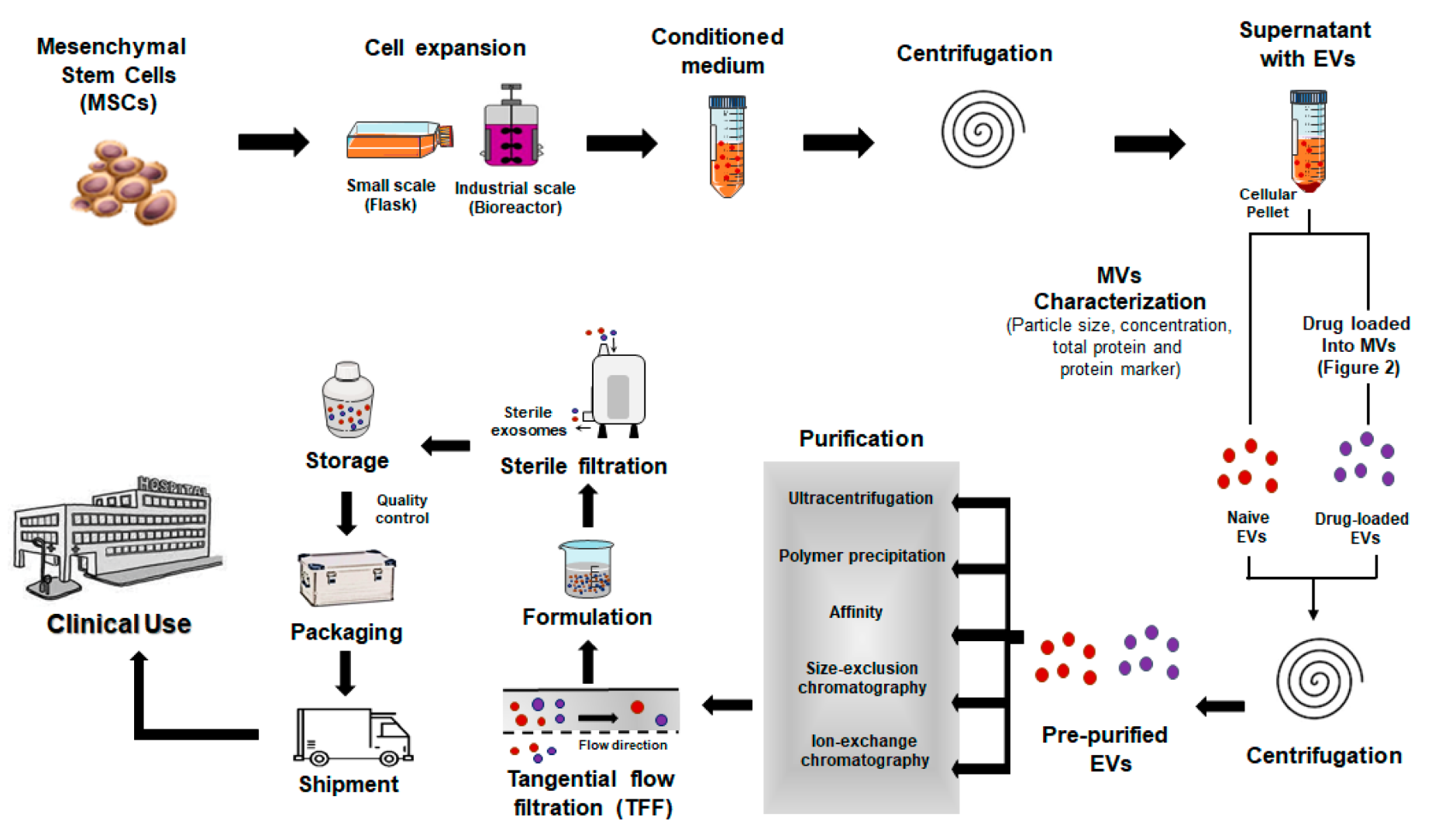
Figure 1. Clinical Manufacturing Approaches to Produce Exosome-based Therapeutics
Why Choose MtoZ Biolabs?
1. Cross-Disciplinary Expertise
Our team combines cell biology, molecular biology, and bioinformatics to tackle projects from basic R&D to large-scale production.
2. Rigorous Quality and Regulatory Support
Comprehensive testing and documentation ensure reproducibility, purity, and compliance with global standards, smoothing the path to clinical or commercial use.
3. Integrated Multi-Omics Analysis
Leveraging genomics, transcriptomics, proteomics, and metabolomics, we provide deep insights into exosome cargo, guiding advanced product design and applications.
4. One-Time-Charge
Our pricing is transparent, no hidden fees or additional costs.
Applications
1. Cell-Free Therapeutics
Large-scale production of exosomes to deliver RNAs, proteins, or drugs in regenerative medicine and immunotherapy.
2. Diagnostics and Biomarker Discovery
Manufacturing exosomes in sufficient quantity for liquid biopsy assays, enabling early disease detection and real-time monitoring.
3. Vaccine Development
Engineered exosomes that present antigenic peptides can boost immune responses, offering innovative vaccine platforms.
4. Cosmetic and Personal Care Formulations
ncorporating exosomes into skincare products to enhance bioactive ingredient delivery and improve skin health.
5. Nutraceutical and Functional Foods
Plant-derived exosomes can be manufactured at scale for use in supplements, promoting gut health and immune modulation.
FAQ
Q: How do you ensure the purity and consistency of manufactured exosomes?
A: We use rigorous isolation methods (e.g., ultracentrifugation, TFF, SEC) and confirm quality through nanoparticle tracking analysis, electron microscopy, and marker profiling to maintain high purity and batch-to-batch consistency.
By offering a robust, scalable platform for high-quality exosome production, MtoZ Biolabs bridges the gap between cutting-edge research and real-world applications. We help you harness the full potential of exosomes—ensuring consistency, safety, and efficacy. If you are interested in our Exosome Manufacturing Service, please feel free to contact us. Our technical specialists are available to provide a free business assessment.
How to order?







