Extracellular Vesicle Isolation and Purification Services
Extracellular vesicles (EVs), including exosomes, microvesicles, and apoptotic bodies, are membrane-bound nanoscale particles secreted by virtually all cell types. They play vital roles in intercellular communication by transporting proteins, nucleic acids, lipids, and metabolites, and have emerged as promising tools in diagnostics, therapeutics, and biomarker discovery. MtoZ Biolabs offers comprehensive Extracellular Vesicle Isolation and Purification Services that deliver high-quality EVs from a wide range of biological sources, including cell culture supernatants, plasma, serum, urine, and plant or microbial systems. Our platform supports both research-grade and preclinical-grade preparations, ensuring the recovery of intact, bioactive vesicles suitable for downstream omics analysis, functional studies, and therapeutic development.
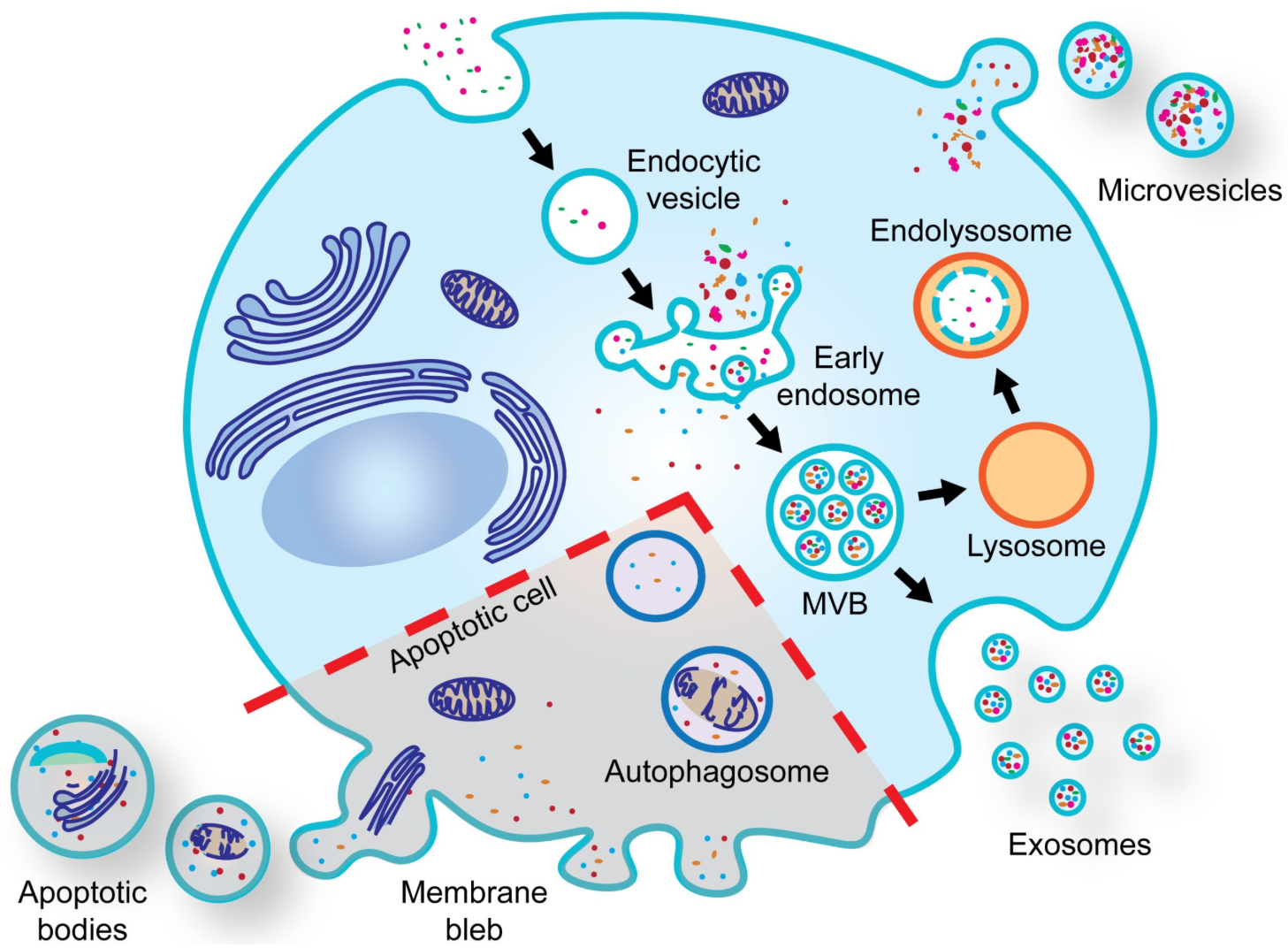
Figure 1. Biogenesis of Extracellular Vesicles
Service at MtoZ Biolabs
MtoZ Biolabs offers a full suite of extracellular vesicle (EV) isolation and purification strategies, tailored to your sample type, downstream application, and desired vesicle subtype. Our core methodologies include: differential centrifugation, density gradient centrifugation, polymer-based precipitation, microfluidic separation, field-flow fractionation, size exclusion chromatography (SEC), tangential flow filtration (TFF), ultrafiltration, and immunoaffinity capture techniques.
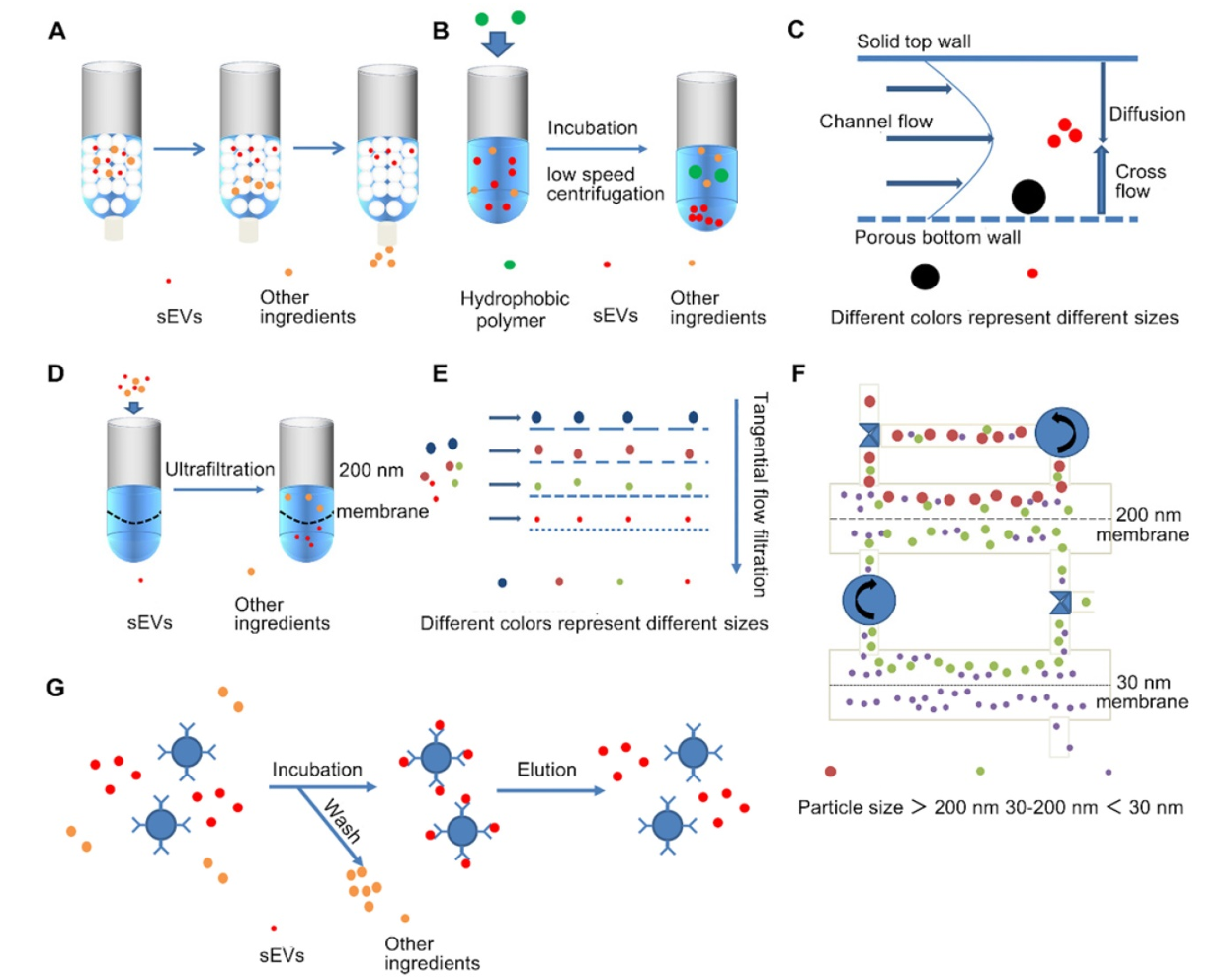
Figure 2. Simplified Diagram of Various sEVs Separation Techniques
By integrating these methods individually or in combination, we ensure flexible, sample-specific solutions for isolating exosomes, microvesicles, or total EVs from diverse matrices. All workflows are performed under standardized conditions with optional vesicle characterization and downstream analysis, enabling high-confidence EV preparation for biomarker discovery, therapeutic development, and multi-omics profiling.
Analysis Workflow
1. Sample Assessment and Preprocessing
The process begins with a thorough assessment of the input sample, including type, volume, storage conditions, and intended downstream application. Samples are then subjected to low-speed centrifugation and filtration steps to eliminate cells, and large debris.
2. Extracellular Vesicle Isolation
Based on the sample characteristics and the desired vesicle population (e.g., total EVs, exosomes, or microvesicles), we select the most appropriate isolation technique. Options include differential centrifugation, density gradient centrifugation, polymer-based precipitation, microfluidics, and more.
3. Extracellular Vesicle Purification
We employ secondary purification steps to minimize residual protein and lipoprotein contaminants, using methods such as size exclusion chromatography (SEC), immunoaffinity capture, field-flow fractionation, and more.
4. Quality Control and Characterization
All isolated vesicles undergo comprehensive quality assessment to verify batch consistency and functional suitability. Standard characterization includes nanoparticle tracking analysis (NTA) for particle size and concentration, transmission electron microscopy (TEM) for morphology, and Western blot, ELISA, or flow cytometry for surface marker verification. Molecular profiling, such as RNA content analysis by RT-qPCR or proteomic analysis via LC-MS/MS, is available upon request.
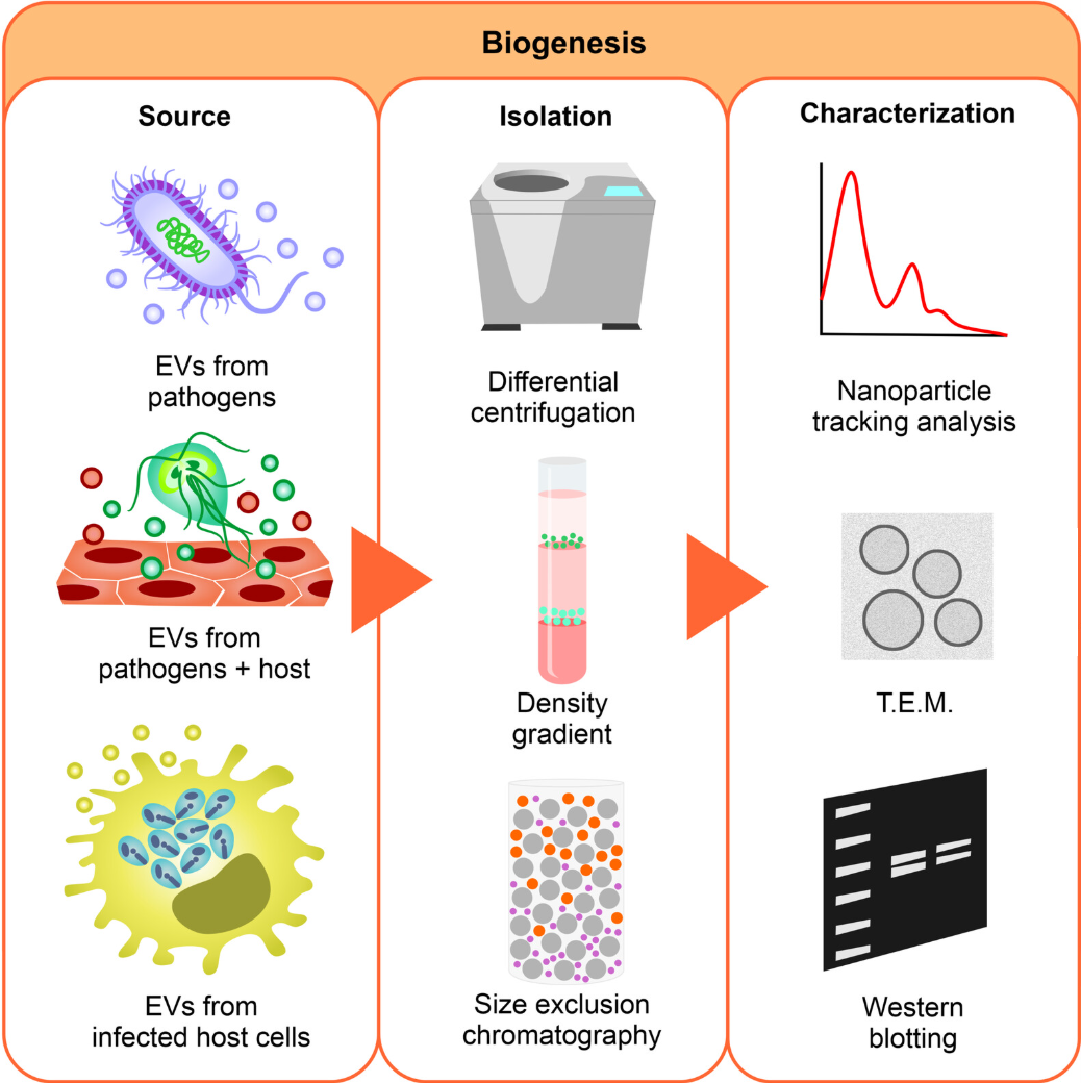
Figure 3. Workflow for Extracellular Vesicle Isolation, Purification, and Characterization
Why Choose MtoZ Biolabs?
☑️Advanced Isolation Technologies: We integrate ultracentrifugation, SEC, TFF, immunoaffinity, and other cutting-edge methods to ensure efficient and application-specific EV isolation and purification.
☑️Strict Quality Control Standards: Every batch undergoes comprehensive characterization, including NTA, TEM, surface marker profiling, and optional omics-based cargo validation.
☑️High Purity: Multi-step workflows ensure removal of contaminants and preservation of vesicle integrity, enabling reliable results in sensitive downstream applications.
☑️Customizable Services: We design isolation and purification workflows tailored to your specific sample type, EV subtype, yield requirement, and downstream application.
Applications
1. Disease Biomarker Studies
Isolated EVs from clinical samples (e.g., plasma, CSF) are suitable for RNA/protein profiling to identify diagnostic or prognostic biomarkers.
2. Extracellular Vesicle Engineering
Isolated EVs serve as clean starting material for surface modification, ligand display, or therapeutic cargo loading in targeted delivery system development.
3. Exosome-Based Vaccine Research
Purified vesicles can be used as natural antigen carriers or delivery platforms in cancer immunotherapy and infectious disease vaccine design.
4. Cellular Communication Studies
EVs isolated from specific cell types enable investigation of intercellular signaling pathways under physiological or pathological conditions.
FAQ
Q1: Can your EV preparations be used for downstream RNA-seq or proteomics?
Yes. Our protocols are designed to preserve vesicle cargo integrity. Isolated EVs are compatible with downstream transcriptomic (e.g., small RNA-seq, mRNA microarray), proteomic (LC-MS/MS), and lipidomic workflows.
High-quality extracellular vesicle isolation and purification are the foundation for all downstream EV research and development. MtoZ Biolabs combines proven isolation technologies with stringent quality control to deliver high-purity, bioactive vesicles tailored to your specific needs. Contact us to explore tailored solutions for your research.
How to order?







