Exosome Cargo Pre-loading Service
Exosome Cargo Pre-loading Service enables the active encapsulation of target molecules—such as RNA, proteins, or small-molecule drugs—into exosomes during their biogenesis by engineering the parent (donor) cells. This strategy is particularly suitable for in vivo delivery studies involving RNA species, recombinant proteins, and small-molecule compounds.
As the applications of exosomes expand across drug delivery, gene regulation, and intercellular communication, achieving precise cargo encapsulation within exosomes has become a pivotal aspect of exosome engineering. Pre-loading refers to the process of genetically or chemically modifying exosome-producing cells to ensure that desired functional molecules are actively packaged into exosomes during their synthesis and release. Compared to post-loading strategies, pre-loading offers higher encapsulation efficiency, improved stability, and better physiological relevance.
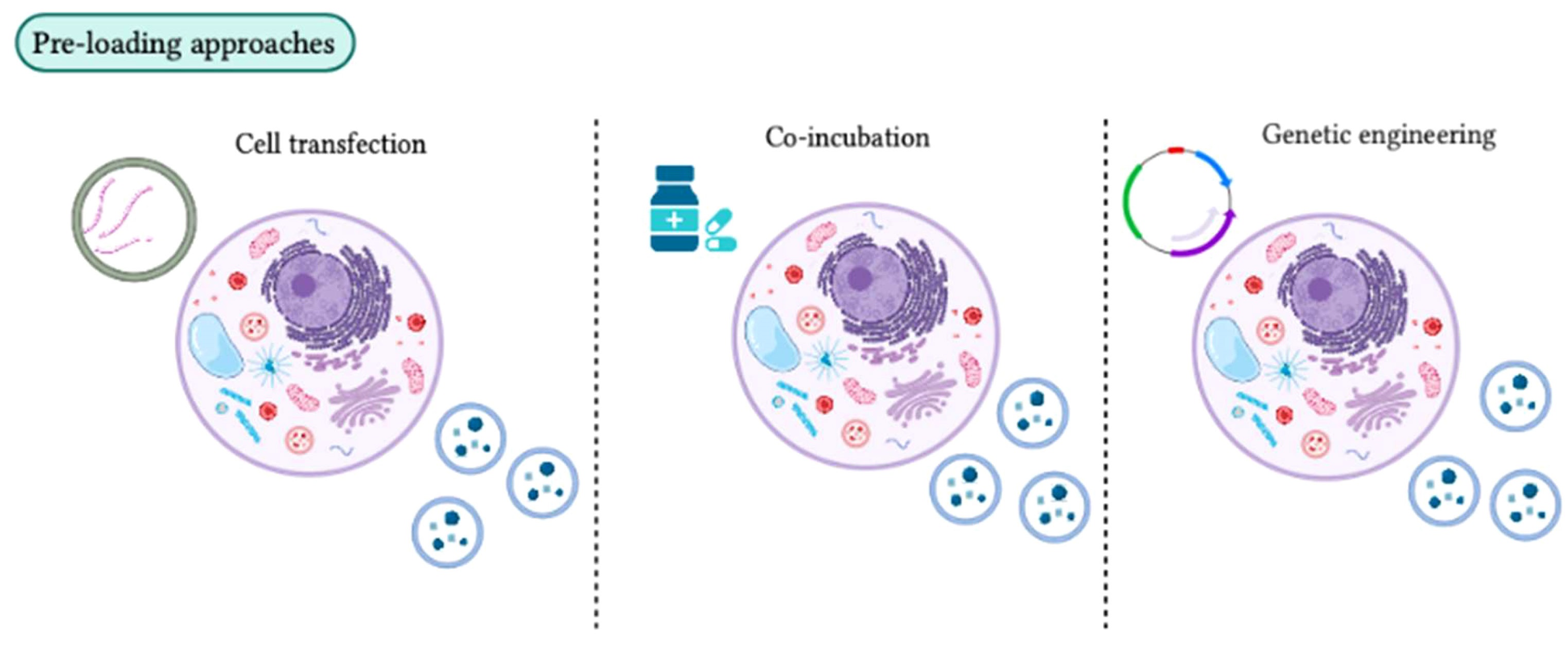
Habib A. et al. Frontiers in Immunology. 2023.
Common methods for Exosome Cargo Pre-loading include:
1. Genetic Engineering Approach
Introduction of plasmids encoding the target RNA or protein into donor cells. To enhance encapsulation efficiency, cargo can be fused with exosomal marker proteins (e.g., CD63-GFP, LAMP2B-cargo).
2. Chemical Induction
Co-incubation of small-molecule drugs or fluorescent probes with donor cells, allowing cellular uptake and partial incorporation into secreted exosomes.
Leveraging a well-established cell engineering system and advanced exosome purification platform, MtoZ Biolabs offers Exosome Cargo Pre-loading Service to support the encapsulation of various molecules including siRNA/miRNA/mRNA, lncRNA, proteins, and fluorescent probes, to facilitate research in targeted therapy, mechanistic studies, and translational applications.
Analysis Workflow
The general workflow of Exosome Cargo Pre-loading Service is as follows:
1. Donor Cell Selection and Engineering
Choose from standardized cell lines such as HEK293T, MSCs, or immune cells, or use customer-provided cell lines.
Transfect or infect donor cells to achieve high intracellular expression of target RNA or proteins.
2. Exosome Induction and Secretion Optimization
Optimize culture conditions to enhance exosome yield and cargo enrichment efficiency.
3. Exosome Isolation and Purification
Isolate high-purity exosomes using ultracentrifugation, size-exclusion chromatography (SEC), or immunoaffinity-based methods.
4.Cargo Loading Verification
RNA: qPCR and small RNA sequencing to assess encapsulation efficiency.
Protein: Western blot, ELISA, or mass spectrometry.
Small molecules/probes: Fluorescence quantification, HPLC, or nanoparticle tracking analysis (NTA).
Applications
Examples of applications for Exosome Cargo Pre-loading Service:
Development of RNA Delivery Systems
For gene silencing and regulation studies in tumors, viral infections, and autoimmune diseases.
Recombinant Protein/Immune Factor-Loaded Exosomes
Engineering exosomes with specific immune or regenerative functions for applications in tissue repair and immunomodulation.
Small Molecule Drug Delivery via Exosomes
Creating sustained-release delivery platforms to improve drug bioavailability and tissue targeting.
Disease Modeling and Target Discovery
Constructing exosomes loaded with specific molecular markers for mechanistic studies and in vivo/ex vivo tracing experiments.
FAQ
Q. What are the advantages of exosome cargo pre-loading compared to post-loading?
Compared to post-loading strategies, pre-loading offers several advantages, including higher cargo stability, more uniform distribution within exosomes, and greater loading efficiency. Additionally, pre-loaded exosomes tend to have better bioavailability and therapeutic efficacy.
Q. Is cargo loading efficiency controllable and quantifiable?
Yes, cargo loading efficiency in pre-loading strategies is both controllable and measurable. We perform quantitative analysis using qPCR for nucleic acids, ELISA or mass spectrometry for proteins, Fluorescence quantification or HPLC for small molecules. By optimizing vector design, expression levels, fusion tags, and donor cell type, we can significantly enhance loading efficiency. Clients receive a detailed report on loading efficiency to ensure data accuracy and reproducibility.
Case Study
In this study, dendritic cells (DCs) were transduced with an adenoviral vector to overexpress the immunomodulatory factor FOXP3. During exosome biogenesis, FOXP3 protein was endogenously packaged into exosomes. The resulting FOXP3 exosomes (FOXP3-EXOs) significantly inhibited pro-inflammatory responses in CD4⁺ T cells, promoted the expansion of Treg cells, and effectively alleviated clinical symptoms in an EAE mouse model, reducing inflammatory infiltration and demyelination. These findings demonstrate the immunosuppressive and disease-ameliorating effects of FOXP3-EXOs, highlighting a novel exosome-based delivery strategy for autoimmune disease therapy.
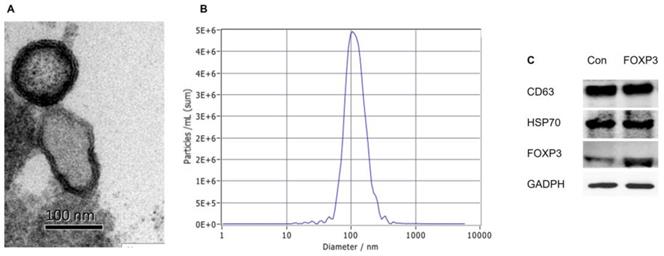
Jia Z. et al. Int J Med Sci. 2022.
How to order?







