Enhancing Tumor Phagocytosis of Multi-antibody-modified Exosome Service
- Optimization of tumor immunotherapy: Improving the clearance efficiency of tumor-associated macrophages (TAMs).
- Microenvironment modulation in CAR-T therapy: Synergistically activating antigen presentation and immune response.
- Combination with immune checkpoint blockade (ICB): Enhancing both phagocytosis and immune activation within the tumor microenvironment.
- Personalized tumor exosome vaccine design: Enhancing adjuvant effects and antigen presentation.
- Phagocytosis signaling mechanism model development: Supporting basic research and target validation.
Enhancing Tumor Phagocytosis of Multi-antibody-modified Exosome Service aims to enhance macrophage-mediated recognition and engulfment of tumor cells by integrating multiple specific antibodies onto the surface of exosomes. This service encompasses the full process—from exosome isolation and purification to antibody modification and functional validation—supporting clients in advancing exosome-based immunotherapy research and applications.
In the field of anti-tumor immunotherapy, macrophage-mediated phagocytosis of tumor cells is a critical component of the innate immune response. However, tumor cells frequently evade immune clearance by expressing "don't eat me" signals such as CD47. Therefore, developing delivery systems that can effectively activate pro-phagocytic signaling and overcome tumor immune evasion mechanisms has become a key strategy in next-generation cancer immunotherapy.
Tumor cells often overexpress CD47, which interacts with the signal regulatory protein alpha (SIRPα) receptor on macrophages to inhibit their phagocytic activity. By engineering exosomes to display anti-CD47 and anti-SIRPα antibodies on their surface, the CD47–SIRPα signaling axis can be effectively blocked, thereby releasing the inhibitory brake on macrophages and enhancing tumor cell phagocytosis.
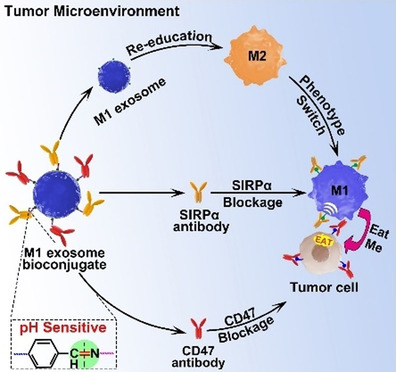
Nie W. et al. Angew Chem Int Ed Engl. 2020.
Leveraging advanced exosome engineering platform, MtoZ Biolabs provides the Enhancing Tumor Phagocytosis of Multi-antibody-modified Exosome Service to modify immune regulatory molecules on the surface of exosomes, build an intelligent immune intervention platform, improve the recognition and phagocytosis efficiency of macrophages on tumor cells, and provide reliable technical support for tumor immunotherapy.
Analysis Workflow
Enhancing Tumor Phagocytosis of Multi-antibody-modified Exosome Service provided by MtoZ Biolabs primarily utilizes genetic engineering and chemical conjugation strategies. The main steps of the chemical conjugation strategy are as follows:
1. Exosome Isolation and Purification
Exosomes are isolated and purified from donor cell culture supernatants using ultracentrifugation, density gradient centrifugation, or size exclusion chromatography (SEC) to ensure high purity and concentration.
2. Multi-specific Antibody Conjugation
Pre-prepared antibodies such as anti-CD47 and anti-SIRPα are directly conjugated to the exosome surface via click chemistry or polyethylene glycol (PEG)-mediated coupling, ensuring directional display and functional activity of antibodies.
3. Characterization of Modified Exosomes
Transmission electron microscopy (TEM), nanoparticle tracking analysis (NTA), dynamic light scattering (DLS), and Western blot are used to comprehensively analyze the size, morphology, surface charge, and antibody display of the modified exosomes, ensuring their quality and consistency.
4. Functional Validation (Optional)
In vitro and in vivo models are used to evaluate the biological functions of the antibody-modified exosomes, including enhancement of macrophage phagocytosis of tumor cells, immune regulatory activity, and antitumor efficacy.
Applications
Enhancing Tumor Phagocytosis of Multi-antibody-modified Exosome Service is suitable for the following research and development applications:
Service Advantages
1. Flexible multi-antibody/multi-protein modification strategies: Supports co-modification with molecules such as anti-CD47, anti-SIRPα, and calreticulin (CRT) via genetic fusion or chemical conjugation. Ensures precise targeting and functional retention.
2. Customizable exosome sources for diverse applications: Offers exosomes derived from HEK293T, dendritic cells, M1 macrophages, etc., to meet needs in tumor immunity, antigen presentation, and microenvironment modulation.
3. Comprehensive quality control and functional validation: Full data support including size (NTA), morphology (TEM), markers (WB), antibody modification rate, and phagocytosis enhancement assays.
4. Supports personalized development and combination strategies: Customizable to fit specific research goals, and can be integrated with ICB, CAR-T, or other therapies to promote translational research.
5. One-Time-Charge: Our pricing is transparent, with no hidden fees or additional costs.
FAQ
Q. Can the Phagocytosis-Enhancing Effect of Modified Exosomes be Validated in Vitro?
Yes. Co-culture systems are commonly used by incubating modified exosomes with tumor cells (e.g., A549, MCF-7) and macrophages derived from human or mouse cells (e.g., THP-1-derived M0/M1 or RAW264.7). Tumor cells are labeled with fluorescent dyes, and phagocytosis efficiency can be quantified via flow cytometry or confocal microscopy.
Q. Which Modification Strategy is Used? Between Genetic Fusion and Chemical Conjugation, which Provides Better Antibody Positioning and Functional Retention?
We offer two strategies: 1. Genetic engineering (fusion of antibodies or proteins to membrane anchors such as Lamp2b or CD63). 2. Chemical conjugation (e.g., click chemistry, PEG-linkers).
Genetic fusion is ideal for achieving structural stability and batch consistency, suitable for long-term studies. Chemical conjugation offers greater flexibility for rapid or multiplex modifications. Functional assays are used to confirm proper localization and activity in both cases.
Case Study
In this study, a pH-responsive nanobioconjugate system was constructed on the surface of M1-derived exosomes. Anti-CD47 and anti-SIRPα antibodies were attached to the exosome membrane via pH-sensitive linkers, allowing their release in acidic tumor microenvironments. This effectively blocked the CD47–SIRPα pathway and enhanced macrophage phagocytosis of tumor cells. Results demonstrated that these engineered exosomes not only significantly boosted antitumor immune responses in vivo but also exhibited excellent tumor inhibition effects in mouse models, offering a novel strategy for synergistic cancer immunotherapy.
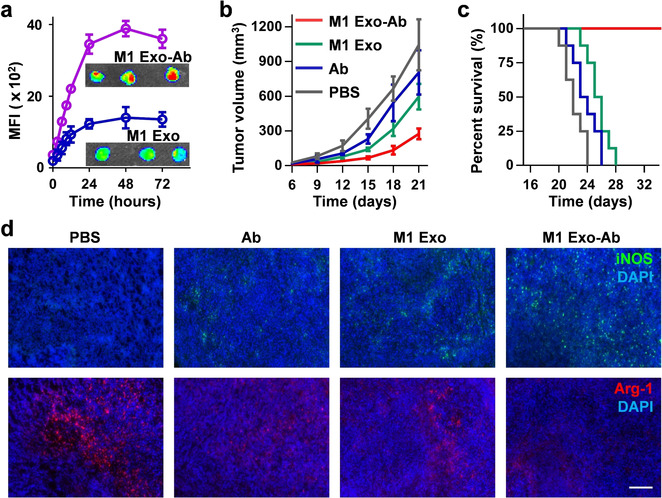
Nie W. et al. Angew Chem Int Ed Engl. 2020.
How to order?







