Electron Paramagnetic Resonance (ESR/EPR) Spectroscopy Analytical Service
Electron Paramagnetic Resonance (EPR/ESR) spectroscopy is a highly sensitive spectroscopic technique specifically used to study systems containing unpaired electrons. Its basic principle is that when a sample is placed in an external magnetic field, the spins of the unpaired electrons undergo resonance absorption at a specific microwave radiation frequency, thereby generating characteristic signals. By analyzing these signals, information such as the electronic environment, molecular structure, and local magnetic field distribution can be obtained. It is widely applied in detecting free radicals and metal ions, supporting the investigation of oxidative stress, disease mechanisms, and the interactions between drugs and reactive species, thereby assisting drug development and quality control.
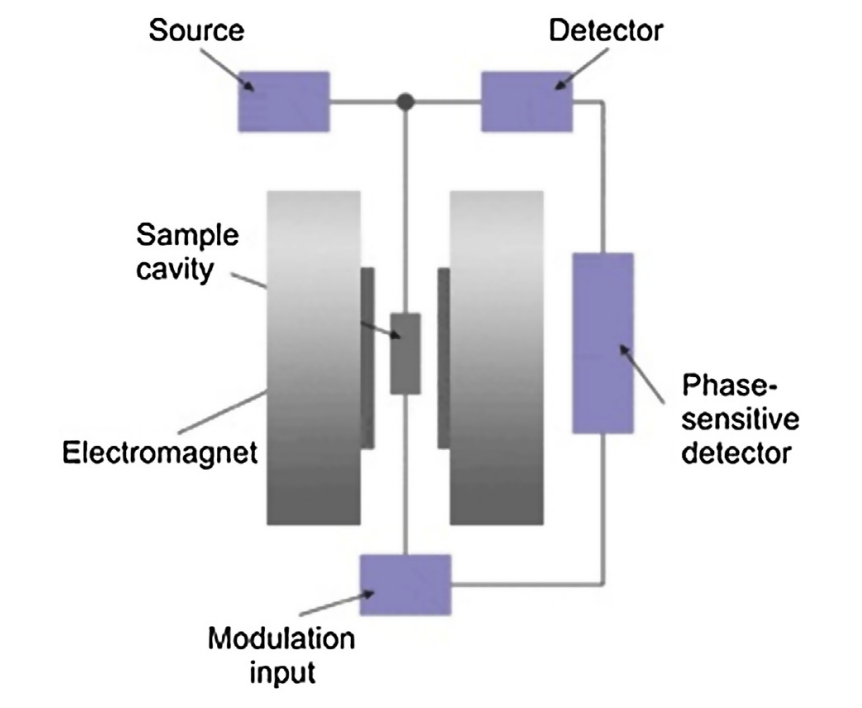
Khulbe, K.C. et al. Membrane Characterization, 2017.
Figure 1. Block Diagram for a Typical Electron Paramagnetic Resonance Spectrometer
Services at MtoZ Biolabs
Based on the advanced electron paramagnetic resonance spectrometer platform, MtoZ Biolabs has launched the electron paramagnetic resonance (ESR/EPR) spectroscopy analytical service which enables highly sensitive detection and quantitative analysis of free radicals, transition metal ions, and paramagnetic centers in samples. By measuring the energy level transitions of unpaired electrons under an external magnetic field and microwave radiation, precise information on the electronic environment, local structure, and molecular interactions can be obtained. The final output data include free radical concentration, electron spin properties, metal ion coordination states, and their dynamic changes in different environments, providing reliable support for drug development, disease mechanism studies, and quality control.
Analysis Workflow
1. Sample Preparation
Select samples containing unpaired electrons, or introduce free radicals or paramagnetic centers through specific methods to ensure the samples meet analytical requirements.
2. Experimental Condition Setup
Place the samples in a sealed and controlled environment, adjusting temperature and atmospheric conditions to ensure stable and reliable signals.
3. Magnetic Field and Microwave Interaction
Under an external constant magnetic field, use microwave radiation of a specific frequency to excite unpaired electrons to undergo energy level transitions, generating detectable resonance effects.
4. Signal Acquisition
By monitoring the changes in energy absorption and emission, record and generate characteristic EPR spectra.
5. Data Analysis and Result Output
Analyze the position, shape, and intensity of the absorption peaks in the spectra to obtain information on electron spin characteristics, coordination environment, and free radical concentration, and finally provide a complete analytical report.
Sample Submission Suggestions
1. Sample Type
Suitable for samples containing unpaired electrons, such as free radicals, transition metal ion compounds, biomacromolecules, or composite materials. Samples may be in solid, liquid, or powder form.
2. Sample Purity
It is recommended to minimize impurities and interfering components to avoid affecting signal quality. For complex samples, the necessary pretreatment should be performed to improve data reliability.
3. Sample Storage
Samples should be stored under dry, light-protected, and appropriate temperature conditions to prevent oxidation or degradation that could weaken signals or cause abnormal spectra.
4. Sample Transportation
Samples should be transported in sealed packaging, and when necessary, under low temperature or inert gas conditions to ensure stability and integrity before reaching the testing platform.
Note: Please indicate whether the sample is magnetic, as this may affect the feasibility of the test. Strongly magnetic samples may damage the instrument; please contact us in advance for confirmation.
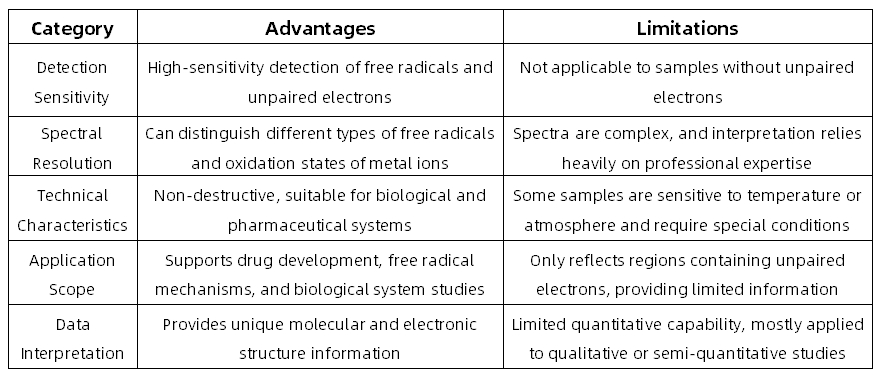
Advantages and Limitations
Applications
1. Drug Development
Electron paramagnetic resonance (ESR/EPR) spectroscopy analytical service can be used to study free radical intermediates in drug molecules and their reaction mechanisms, supporting new drug design and optimization.
2. Antioxidant Evaluation
By detecting free radical scavenging efficiency, it evaluates the activity and stability of antioxidants in drugs and nutritional supplements.
3. Disease Mechanism Research
ESR/EPR can analyze changes in free radical levels in cells or tissues, helping to reveal the role of oxidative stress in the occurrence and development of diseases.
4. Biomaterial Analysis
By detecting free radical reactions in biomaterials under in vivo or in vitro conditions, it evaluates their biocompatibility and long-term stability.
5. Quality Control
Electron paramagnetic resonance (ESR/EPR) spectroscopy analytical service can be applied to monitor residual free radicals in drug formulations, ensuring production safety and product stability.
FAQs
Q1: Can Non-radical Samples Be Detected?
A1: If the sample does not contain unpaired electrons, ESR/EPR cannot obtain valid signals. However, in some cases, methods such as adding spin-trapping agents can be used to “fix” transient free radicals, thereby enabling indirect detection.
Q2: What Are the Requirements for Sample Purity and Stability?
A2: Samples should minimize impurity interference and maintain chemical stability to ensure clear and reliable signals. For samples that are easily oxidized or temperature-sensitive, it is recommended to store and transport them under an inert atmosphere or low-temperature conditions.
Q3: What Are the Advantages of ESR/EPR compared to Other Spectroscopic Methods?
A3: Compared with infrared, ultraviolet, or NMR spectroscopy, ESR/EPR is more sensitive to free radicals and paramagnetic centers. It can directly detect these special molecules, making it suitable for studying short-lived or low-concentration species that conventional methods find difficult to capture.
How to order?







