Combinational Applications of Cryo-EM, X-ray Crystallography and NMR
Cryo-electron microscopy (Cryo-EM), X-ray crystallography, and nuclear magnetic resonance (NMR) are the three core technologies for elucidating biomacromolecular structures. Each has its own strengths in resolution, applicable sample states, and acquisition of dynamic information, collectively forming a multi-scale and complementary structural biology framework. Cryo-EM is suitable for analyzing large macromolecular complexes and samples that are difficult to crystallize, providing three-dimensional structures under near-native conditions. X-ray crystallography offers the highest atomic resolution and is ideal for highly ordered crystalline samples. NMR enables the study of protein dynamics and flexible regions in solution. Through their combined application, these techniques not only enhance the accuracy and completeness of structural determination but also compensate for the limitations of individual methods, enabling a comprehensive understanding from static structures to dynamic mechanisms.
The combinational applications of Cryo-EM, X-ray crystallography, and NMR have been widely applied in cutting-edge fields such as membrane protein structure analysis, signal transduction complex studies, multi-conformational state identification, and drug binding mechanism elucidation. In antibody drug development, protein-protein interaction research, and complex target validation, the integration of these three techniques provides a complete solution from conformational diversity to structural refinement, significantly advancing structure-function relationship studies and structure-based drug design.
Services at MtoZ Biolabs
Based on the three major platforms of structural analysis—Cryo-EM, X-ray crystallography, and nuclear magnetic resonance (NMR), the combinational applications of Cryo-EM, X-ray Crystallography and NMR service provided by MtoZ Biolabs utilizes advanced cryogenic electron microscopes, high-throughput X-ray diffractometers, and high-sensitivity NMR spectrometers to respectively obtain the overall architecture of macromolecular complexes, crystal-level atomic coordinates, and dynamic structural information in solution. This service, built on integrated multi-technology approaches, ensures the acquisition of multidimensional data with complete conformational coverage, high resolution, and strong structural consistency, supporting precise analysis and mechanistic studies of complex protein systems.
Analysis Workflow
1. Sample Preparation and Quality Assessment
Prepare high-purity protein samples tailored to the requirements of Cryo-EM, X-ray crystallography, and NMR, followed by evaluation of purity, stability, and aggregation status.
2. Multiplatform Structural Data Acquisition
Use Cryo-EM to capture the overall architecture of macromolecular complexes, X-ray crystallography to resolve high-resolution 3D structures in the crystalline state, and NMR to obtain structural and dynamic information in solution.
3. Data Integration and Structural Reconstruction
Integrate datasets from each technique to build a high-fidelity composite structural model through modeling, fitting, and cross-validation, thereby overcoming the limitations of any single method.
4. Structural Annotation and Mechanistic Analysis
Perform in-depth analysis of key domains, interaction interfaces, and conformational changes to provide structural insights into functional mechanisms, drug target design, and molecular regulation.
Service Advantages
1. Comprehensive Structural Coverage
MtoZ Biolabs integrates Cryo-EM, X-ray crystallography, and NMR platforms to achieve multi-scale structural analysis of proteins, ranging from low-resolution conformations to atomic-level detail.
2. High Versatility
Techniques are flexibly selected based on sample type, making the service suitable for difficult-to-crystallize, highly flexible, or large macromolecular samples, ensuring comprehensive and accurate structural information.
3. Reliable Data
By combining cross-validation and integrated modeling, the quality of 3D reconstruction is significantly enhanced, improving the accuracy and stability of structural analysis.
4. One-Stop Service
End-to-end support is provided, from sample preparation to structural modeling, facilitating mechanism studies, target discovery, and new drug development.
Applications
1. Macromolecular Structure Analysis
Combinational applications of Cryo-EM, X-ray crystallography, and NMR are suitable for high-resolution structural analysis of macromolecular systems such as protein complexes, membrane proteins, and nucleic acids, compensating for the limitations of individual techniques in resolution or sample adaptability.
2. Dynamic Conformational Studies
NMR captures molecular dynamics in solution, while Cryo-EM and X-ray provide stable conformation information, supporting studies on protein conformational changes and functional regulation mechanisms.
3. Drug Mechanism Elucidation
Combinational applications of Cryo-EM, X-ray crystallography, and NMR can precisely reveal binding sites and structural changes of drugs such as small molecules and antibodies with their targets, aiding lead compound optimization and pharmacodynamic prediction.
4. Multi-Scale Structural Integration Modeling
Cryo-EM provides global architecture, X-ray contributes atomic-level detail, and NMR supplements flexible region information, enabling the construction of high-precision integrative models.
Case Study
1. Integrative Approaches in Structural Biology: A More Complete Picture from the Combination of Individual Techniques
This study aims to explore the significance of integrative strategies in structural biology by combining cryo-electron microscopy (Cryo-EM), X-ray crystallography, and nuclear magnetic resonance (NMR) to more comprehensively resolve the structures and dynamic features of biomacromolecular complexes. The research targets include various complex proteins and their assemblies. Through literature cases and methodological comparisons, the authors analyze the strengths and limitations of each technique and demonstrate how data integration can overcome structural blind spots inherent to single-method approaches. The results show that multi-technique complementarity provides higher-resolution and more complete structural models while revealing flexible regions and functional conformations. The conclusion indicates that the future of structural biology will move toward multi-platform integrative analysis, where combining information across different scales and resolutions is essential for accurate structural elucidation and understanding biological mechanisms.
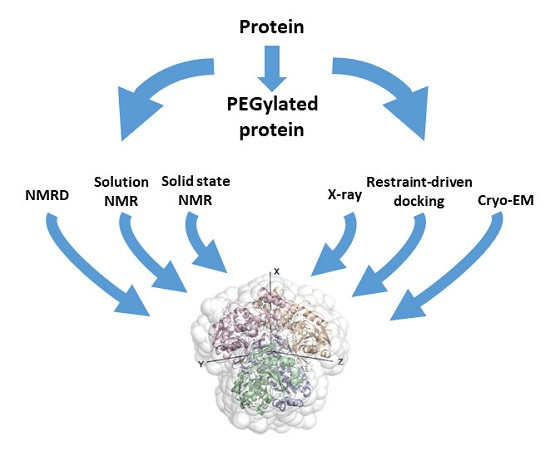
Cerofolini, L. et al. Biomolecules, 2019.
Figure 1. The Graphical Abstract of this Study.
FAQ
Q1: What Types of Projects Are Suitable for the Combined Use of Cryo-EM, X-ray Crystallography, and NMR?
A1: The integrated approach is ideal for complex macromolecular systems that require high-resolution structures, full conformational coverage, and dynamic feature analysis, such as membrane proteins, large assemblies, flexible domains, or regions in Cryo-EM maps with limited resolution.
Q2: Is it Necessary to Use All Three Techniques Simultaneously?
A2: Not necessarily. The combination can be flexibly tailored based on sample characteristics and research goals—for example, Cryo-EM with X-ray for reconstructing stable structures, or Cryo-EM with NMR for capturing dynamic regions.
Q3: What Are the Differences in Sample Requirements among theThree Techniques?
A3: Cryo-EM requires samples with high concentration and homogeneity but does not require crystallization; X-ray crystallography requires high-quality crystals; NMR demands highly pure, stable samples with moderate molecular weight (typically <50 kDa is preferred).
How to order?







