Chiral Compound Enantiomeric Excess (ee) Value Analytical Service
- ee Value analysis by Chiral HPLC
- ee Value analysis by Chiral GC
- ee Value analysis by Circular Dichroism (CD)
- ee Value analysis by NMR
- ee Value analysis by Chiral HPLC/GC-MS
Enantiomeric excess (ee) is a key parameter for evaluating the ratio between two enantiomers in a chiral compound, and it is often referred to as optical purity. Since different enantiomers may exhibit significantly different pharmacological activity or toxicity, accurate determination of ee is essential in drug development, asymmetric synthesis research, and quality control in industrial production. Common analytical methods include chromatographic techniques (such as Chiral HPLC and Chiral GC), spectroscopic techniques (such as CD and NMR), and mass spectrometry-based hyphenated techniques (such as Chiral HPLC/GC-MS). These approaches provide reliable assurance for the purity and safety of chiral compounds.
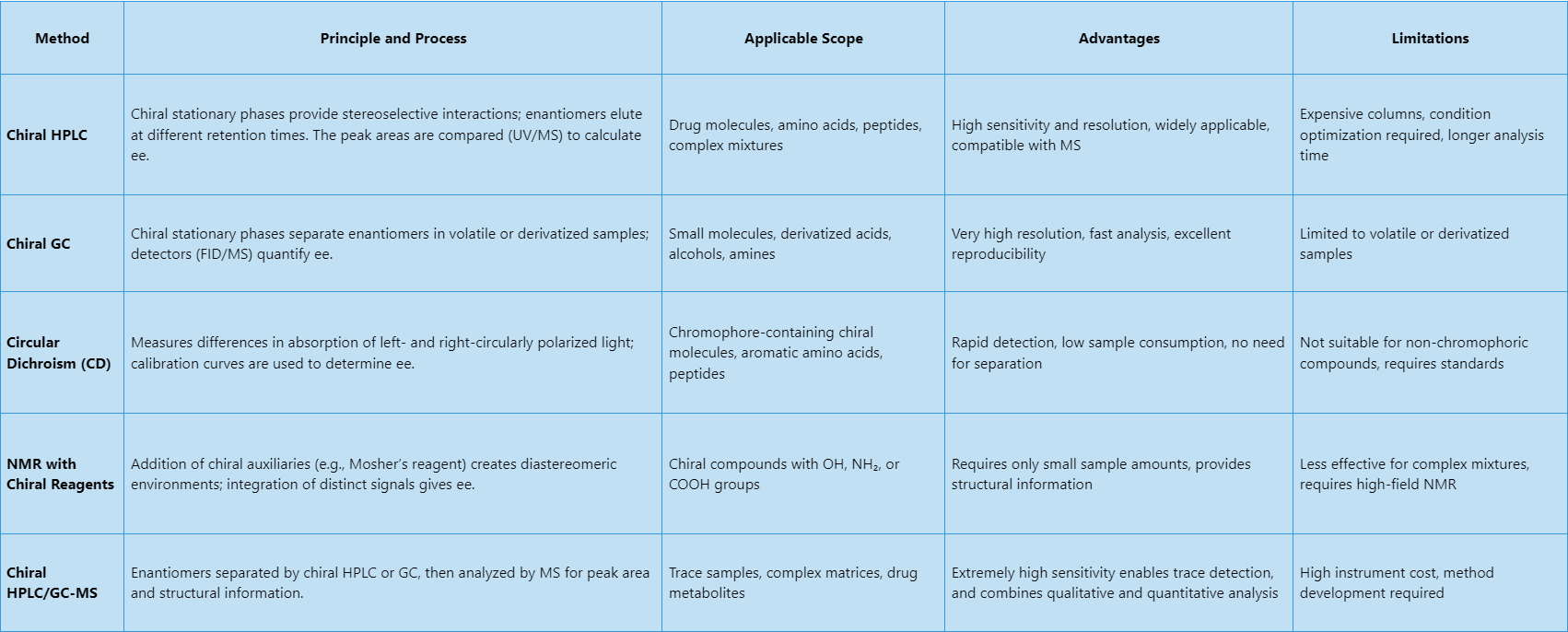
Table1. Enantiomeric Excess (ee) Value Common Analytical Methods
Services at MtoZ Biolabs
MtoZ Biolabs offers Chiral Compound Enantiomeric Excess (ee) Value Analytical Service to accurately evaluate the optical purity of chiral compounds. We employ internationally recognized techniques, including Chiral HPLC, Chiral GC, Circular Dichroism (CD), polarimetry, and NMR with chiral reagents, as well as hyphenated approaches such as HPLC/GC-MS. These methods allow for highly sensitive and high-resolution detection even in complex samples. Our services are widely applicable in drug development, asymmetric catalysis validation, and industrial quality control, providing researchers and enterprises with fast and reliable data to support high-standard compliance throughout drug discovery and production.
Analysis Workflow
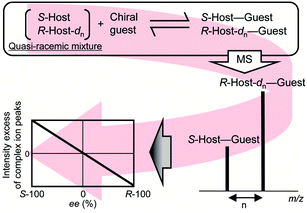
Nakakoji, T. et al. RSC Adv. 2021.
Figure 1. Workflow of Chiral Compound Enantiomeric Excess (ee) Value Analysis by MS
Service Advantages
1. Flexible Multi-Platform Options
MtoZ Biolabs integrates multiple mainstream analytical platforms, including Chiral HPLC, Chiral GC, CD, NMR, and LC/GC-MS, selecting the most suitable method for each sample to ensure accuracy and reliability.
2. High Sensitivity and High Resolution
With advanced chromatography and mass spectrometry systems, we achieve high-resolution separation and trace-level quantitation in complex matrices, delivering precise ee analysis.
3. Wide Applicability
Our chiral compound enantiomeric excess (ee) value analytical service covers a broad range of chiral compounds, including drug molecules, amino acids, peptides, natural products, and metabolites, supporting applications in drug development, asymmetric synthesis, natural product research, and clinical metabolism studies.
4. One-Time Charge
Our pricing is transparent with no hidden fees or additional costs.
Applications
1. Drug Development and Quality Control
During drug development, different enantiomers may show markedly different pharmacological effects or toxicities. ee measurement ensures that the desired enantiomer is obtained with high optical purity and that strict quality control is maintained during production to meet pharmacopeia and regulatory standards.
2. Asymmetric Catalysis and Synthetic Methodology
ee is the key index for evaluating the selectivity of chiral catalysts and the performance of asymmetric reactions. Measuring ee allows researchers to optimize reaction conditions, compare catalysts, and guide the development of novel catalytic systems.
3. Natural Product Isolation and Structural Identification
In natural product chemistry, the chiral compound enantiomeric excess (ee) value analytical service helps assess whether isolated compounds are enantiopure or mixtures, supporting both structure elucidation and biological activity studies.
4. Clinical and Metabolic Research
In pharmacokinetics and in vivo drug analysis, ee measurement reveals differences in the absorption, distribution, metabolism, and excretion of enantiomers, providing critical data for personalized medicine and safety evaluation.
Sample Submission Suggestions
1. Sample Type
Accepts chiral small molecules, amino acids, peptides, natural products, drug intermediates, or metabolites. For complex matrices (such as biological samples), please specify background components or provide pretreatment information.
2. Sample Form
Samples may be submitted as solid powders or solutions. For solutions, please indicate the solvent and approximate concentration.
3. Purity and Stability
Samples should have sufficient purity for accurate measurement. For light-sensitive or unstable compounds, please note special handling requirements so that appropriate protection measures can be applied.
4. Storage and Transport
Samples should be sealed in clean containers to avoid contamination. Depending on chemical properties, transport may be at ambient temperature, refrigerated, or on dry ice. Please include a sample information sheet (compound name, structure, molecular weight, solvent, concentration, and special notes).
For detailed sample submission requirements, please contact MtoZ Biolabs staff. We will provide a complete submission guideline upon request.
With advanced platforms and extensive expertise, MtoZ Biolabs is committed to delivering high-quality chiral compound enantiomeric excess (ee) value analytical service for chiral compounds. Whether for drug development, asymmetric synthesis research, or industrial quality control, we provide reliable data to support your projects.
How to order?







