CD vs NMR vs X-ray: Which Structural Analysis Method is Right for You?
In life sciences research and pharmaceutical development, the structural characterization of biological macromolecules plays a pivotal role. Among the most widely employed techniques are Circular Dichroism (CD), Nuclear Magnetic Resonance (NMR), and X-ray Crystallography, each offering distinct advantages suited to specific experimental contexts. This review outlines the selection criteria for these methods in terms of resolution, sample requirements, applicability, and experimental timeframe.
Circular Dichroism (CD): An Efficient Approach for Rapid Assessment of Secondary Structure
CD spectroscopy infers molecular conformation by detecting differences in the optical activity of chiral molecules, making it particularly suitable for probing the secondary structures of proteins and nucleic acids (e.g., α-helices, β-sheets).
1. Advantages
(1) Rapid and high-throughput: Enables data acquisition within hours, ideal for preliminary structural screening.
(2) Minimal sample requirement: Applicable to microgram-level quantities, with relatively low demands on sample concentration and purity.
(3) Environmental sensitivity: Allows monitoring of conformational changes under varying pH, temperature, and ionic conditions, facilitating studies of protein stability and ligand binding.
2. Limitations
(1) Limited resolution: Provides only overall conformational trends without atomic-level detail.
(2) Restricted structural insight: Inadequate for constructing high-precision three-dimensional models.
CD is particularly advantageous for studies aimed at screening conformational variations, optimizing buffer conditions, or assessing protein folding states, offering an efficient and cost-effective solution.
Nuclear Magnetic Resonance (NMR): Structural and Dynamic Insights in Solution
NMR spectroscopy exploits the resonance behavior of nuclear spins in a magnetic field to deliver high-resolution structural, dynamic, and interaction information for biomolecules under near-physiological conditions.
1. Advantages
(1) Atomic-level resolution: Well-suited for determining the detailed structures of small to medium-sized proteins (typically < 30 kDa).
(2) Dynamic structural characterization: Enables investigation of conformational flexibility, binding mechanisms, and molecular dynamics.
(3) No crystallization required: Conducted directly in solution, preserving physiological relevance.
2. Limitations
(1) High sample demand: Requires millimolar concentrations of highly purified and stable protein samples.
(2) Size constraints: Larger macromolecules suffer from significant spectral overlap, complicating data interpretation.
(3) Technical complexity: Involves costly instrumentation and necessitates highly experienced analysts.
NMR is optimal for examining small proteins, peptides, nucleic acids, or protein–ligand interactions, especially when structural dynamics under native-like conditions are of interest.
X-ray Crystallography: The Established Standard for High-Resolution Structure Determination
X-ray crystallography remains a cornerstone technique for elucidating atomic-resolution structures of proteins, nucleic acids, and their complexes, underpinning many advances in structural biology.
1. Advantages
(1) Atomic-resolution capability: Yields three-dimensional structures with exceptional precision.
(2) Broad applicability: Suitable for protein complexes, macromolecular assemblies, and enzyme–substrate complexes.
(3) Strong database integration: Benefits from extensive structural repositories, expediting homology modeling and functional annotation.
2. Limitations
(1) Crystallization challenges: Optimization of crystallization conditions often requires extensive empirical trials, particularly for flexible or membrane-associated proteins.
(2) Extended experimental duration: The process from sample preparation to final structural refinement may span several weeks or longer.
(3) Static representation: Captures molecules in crystallized states, limiting direct insights into conformational dynamics.
X-ray crystallography is indispensable when highly accurate structural information is required, particularly for drug target design, structure-based drug discovery, and molecular docking studies.
Summary of Method Selection
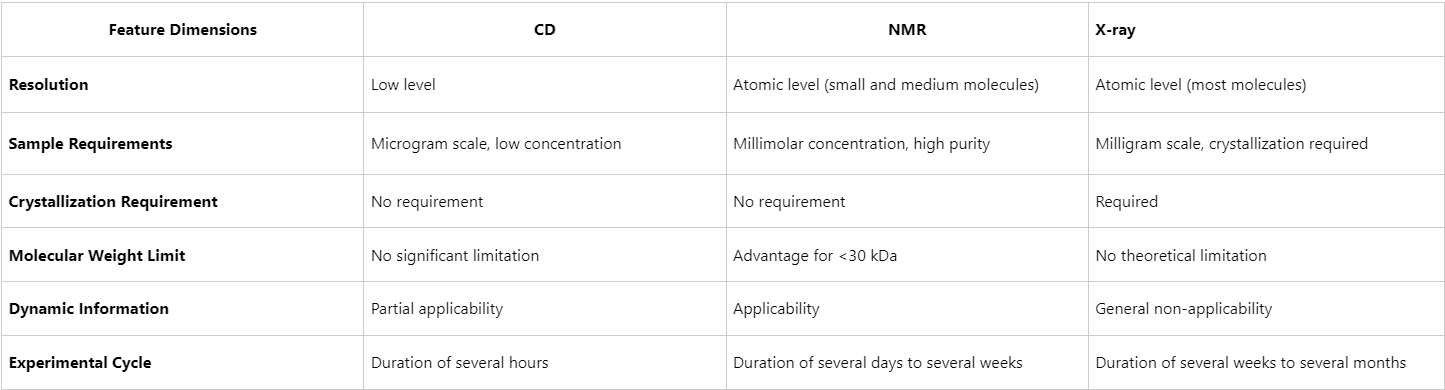
Figuer1. CD vs NMR vs X-ray
Selecting the most appropriate analytical technique represents a critical step in successful structural biology research. Leveraging expertise in protein expression, purification, and structural determination, MtoZ Biolabs provides integrated solutions that enable breakthroughs from preliminary conformational screening to atomic-level resolution. Tailored service packages ensure the delivery of high-quality data to meet the demands of basic research, target validation, and drug discovery programs.
MtoZ Biolabs, an integrated chromatography and mass spectrometry (MS) services provider.
Related Services
How to order?







