Calcifediol Analysis Service
- Qualitative analysis of the metabolites of calcifediol
- Quantitative determination of calcifediol concentration
- Providing basis for research and product development
- Animal tissue ≥ 100 mg
- Plant tissue ≥ 200 mg
- Serum/ Plasma ≥ 200 μL
- Urine ≥ 2 mL
Calcifediol is the primary hydroxylated metabolite of vitamin D, characterized by the presence of a hydroxyl (–OH) group at the 25th carbon position of the cholesterol molecule. Calcifediol is formed in the liver from cholesterol-derived vitamin D (such as vitamin D3 or D2) through the catalytic action of 25-hydroxylase (CYP2R1) and serves as an important endogenous marker in biological systems, reflecting the body's vitamin D status. Calcifediol exhibits significant biological activity in the body, particularly in bone health and calcium-phosphorus metabolism. It is considered an indirect ligand for the nuclear vitamin D receptor (VDR), regulating physiological processes such as calcium absorption, bone mineralization, and immune response through its further conversion into the active form, 1,25-dihydroxyvitamin D (Calcitriol).
By interacting with VDR receptor metabolites, calcifediol can influence various physiological functions, including the regulation of the immune system, cell proliferation, and differentiation. Due to its critical role in calcium-phosphorus metabolism and immune function regulation, calcifediol is regarded as a key biomarker in drug development and health management. Quantitative analysis of calcifediol helps assess an individual's vitamin D nutritional status, aids in diagnosing vitamin D deficiency and related diseases, and provides a basis for personalized treatment. The formation of calcifediol involves multiple enzymatic steps, particularly the catalysis by 25-hydroxylase (such as CYP2R1) in the liver. Quantitative analysis of calcifediol and its metabolites can help reveal the activity of the vitamin D metabolic pathways under different physiological and pathological conditions, explore its changes in disease states, and provide important insights for research on bone metabolic diseases and immune disorders.
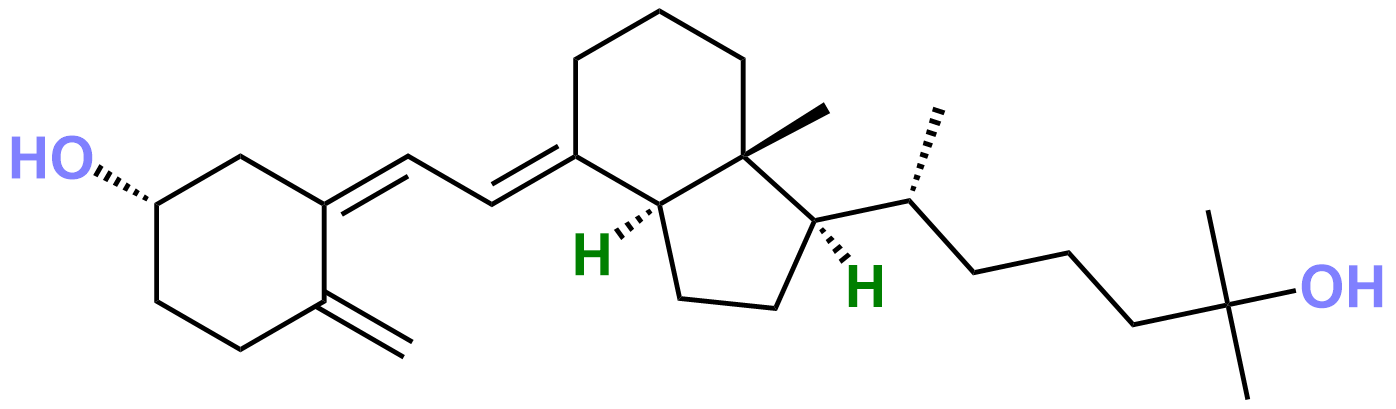
Figure 1. The Structure of Calcifediol
MtoZ Biolabs is pleased to offer an unparalleled Calcifediol Analysis Service, supported by our state-of-the-art liquid chromatography-mass spectrometry (LC-MS) platform and a team of skilled metabolomics specialists. Our service offers an exhaustive analysis of calcifediol, ensuring high precision and sensitivity.
Analysis Workflow
Service Advantages
1. Continuously refined protocols and cutting-edge analytical tools
2. Expert experimental planning
3. Rapid processing times
4. Exceptional precision, selectivity, and sensitivity
Applications
Sample Submission Requirements
1. Sample Types
Serum, plasma, urine, tissues, and other biological samples. A minimum of 3 homogeneous materials for each sample is necessary.
2. Sample Volume
3. Sample Preservation
To ensure stability, samples should be stored at -80°C.
Note: Kindly provide details related to sample collection and handling.
Deliverables
1. Experimental Procedures
2. Relevant Liquid Chromatography and Mass Spectrometry Parameters
3. Detailed Information on Calcifediol
4. Raw Data
5. Custom Analysis Report
With our Calcifediol Analysis Service, we aim to support your research and product development efforts by providing accurate and trustworthy results.
How to order?







