Biomarker Proteomics Service
Biomarker proteomics service aim to analyze the proteome in samples using high-throughput technology to discover potential biomarkers related to diseases or physiological processes. Mass spectrometry technology, as one of the core technologies of modern proteomics, is also one of the most commonly used technology in biomarker proteomics. Through in-depth analysis of protein expression, post-translational modification, protein interaction, etc., mass spectrometry technology can provide technical support for the discovery and verification of biomarkers and their promotion in clinical applications. Based on chromatography and mass spectrometry platforms, MtoZ Biolabs provides biomarker proteomics service dedicated to helping customers identify, verify and quantify potential biomarkers in biomedical research through in-depth analysis of proteins in biological samples.
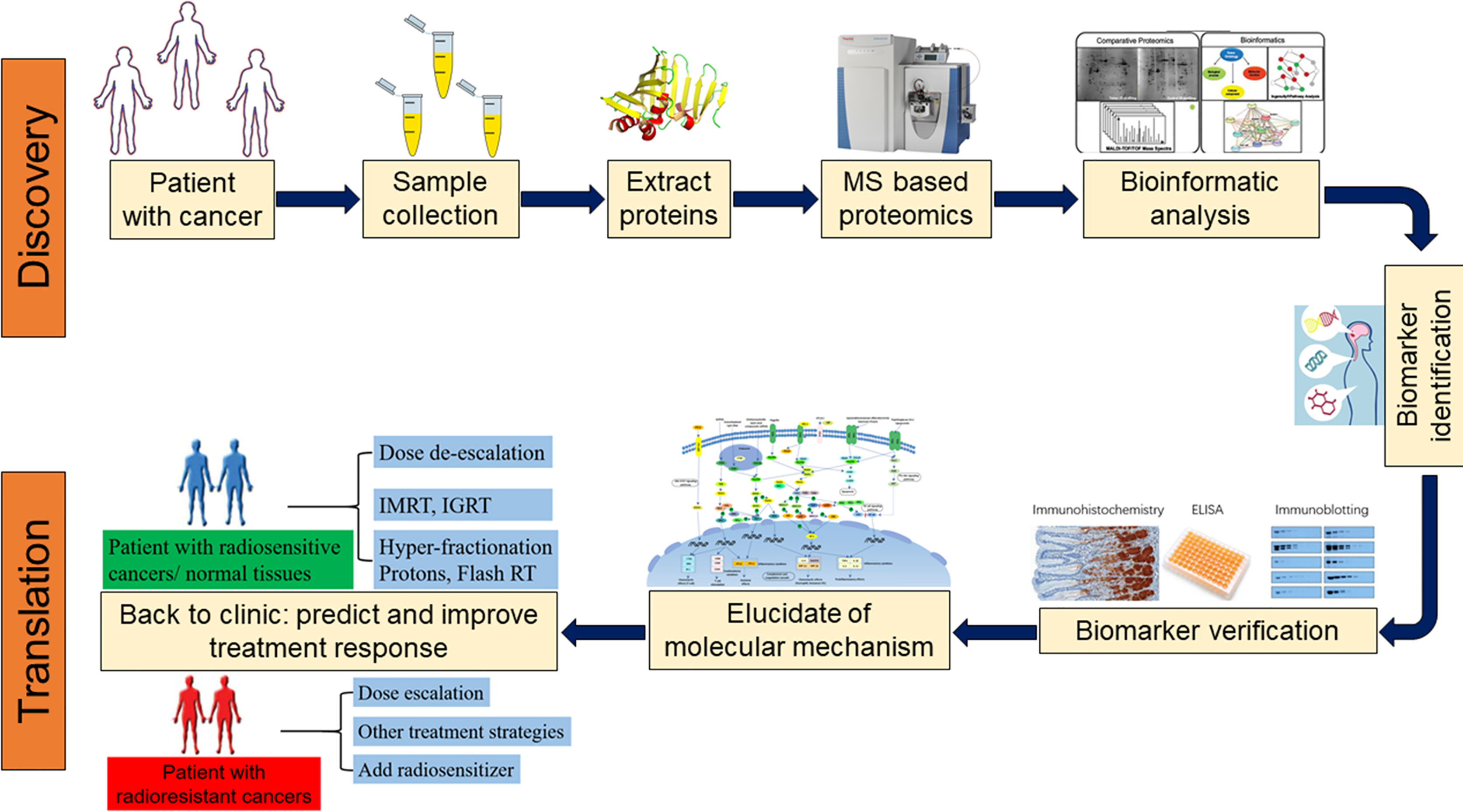
Luo H. et al. Front Oncol. 2022.
Service Advantages
1. Advanced Analysis Platform: MtoZ Biolabs established an advanced biomarker proteomics service platform, guaranteeing reliable, fast, and highly accurate analysis service.
2. One-Time-Charge: Our pricing is transparent, no hidden fees or additional costs.
3. High-Data-Quality: Deep data coverage with strict data quality control. AI-powered bioinformatics platform integrates all biomarker proteomics service data, providing clients with a comprehensive data report.
Applications
Biomarker proteomics service is widely used in the fields of early diagnosis of diseases, prognosis assessment, drug response monitoring and personalized medicine.
Early diagnosis of diseases
Biomarker proteomics service can help achieve early diagnosis by screening disease-related protein markers, especially for diseases such as cancer, cardiovascular disease, and neurodegenerative diseases.
Prognosis assessment
By analyzing the expression patterns and changes of proteins in patients, biomarker proteomics can help discover potential biomarkers related to disease prognosis.
Drug response monitoring
Help monitor patients' responses to drugs and guide drug dosage adjustments and optimization of treatment plans. For example, biomarker proteomics service can screen and verify biomarkers related to adverse drug reactions, help identify potential toxic reactions early, and avoid serious side effects.
Personalized medicine
By analyzing the protein expression characteristics in patients, tailor-made personalized treatment plans for each patient. For example, biomarker proteomics service can help doctors configure personalized interventions based on patients' biomarkers by revealing markers related to metabolic diseases such as diabetes and obesity.
FAQ
Q. How to control sample heterogeneity?
Sample heterogeneity is a common and unavoidable challenge in proteomics studies of clinical samples. The source of samples (such as blood, tissue, urine, etc.) may vary depending on the patient's physiological and pathological status, gender, age, diet, environment, and other factors. This heterogeneity will affect the expression profile of proteins, and thus affect the discovery and validation of biomarkers. In order to effectively control sample heterogeneity, we can adopt the following strategies:
Standardized sampling procedures: Ensure that all sample collection conditions are consistent, such as collection time, collection method (such as the use of anticoagulants, preservation fluids, etc.), sample processing methods, etc. These standardized measures help reduce the impact of external variables on the results.
Use a control group: Set up an appropriate control group, preferably selecting healthy individuals who match the patient group as much as possible in terms of gender, age, lifestyle, etc. This helps to reveal the true biological background of protein differences rather than false positive results caused by external variables.
Stratified analysis: During the analysis stage, samples can be stratified according to the patient's clinical characteristics (such as disease type, disease stage, etc.). This helps to reduce interference caused by disease heterogeneity and can more clearly reveal biomarkers associated with specific diseases or disease stages.
Q. How to control false positives and false negatives in data processing?
In high-throughput proteomics research, the amount of data is huge and complex. In order to minimize false positives and false negatives, the following measures can usually be taken:
Strict data filtering and quality control: Mass spectrometry data often contains a lot of noise and low-quality signals, so strict data preprocessing and quality control are required. For example, use specific software to denoise the raw data, correct drift, correct errors, and remove low-abundance or irrelevant proteins.
Statistical methods and multiple hypothesis testing: Since thousands of proteins are usually measured simultaneously in proteomics experiments, in order to reduce the false positives caused by multiple hypothesis testing, we can use appropriate statistical methods for correction, such as the Benjamini-Hochberg (BH) method, to control the false discovery rate (FDR). In addition, the use of more stringent P value thresholds can also help reduce false positives.
Validation experiments: The screening results of candidate biomarkers need to be verified by independent experiments. Common methods include Western blot, ELISA, immunohistochemistry, etc. These validation experiments can help confirm whether the differential proteins found in mass spectrometry analysis have actual biological significance.
Biological replication and cross-platform validation: By increasing biological replication and validating the results on different experimental platforms (such as using different mass spectrometry platforms or different sample processing methods), the reliability of the results can be effectively improved and errors can be reduced.
Case Study
The study conducted proteomic analysis on the plasma of patients, revealed potential biomarkers of the diseases through bioinformatics analysis, and verified them through enzyme-linked immunosorbent assay. The accuracy of diagnosis was improved by constructing a biomarker combination.
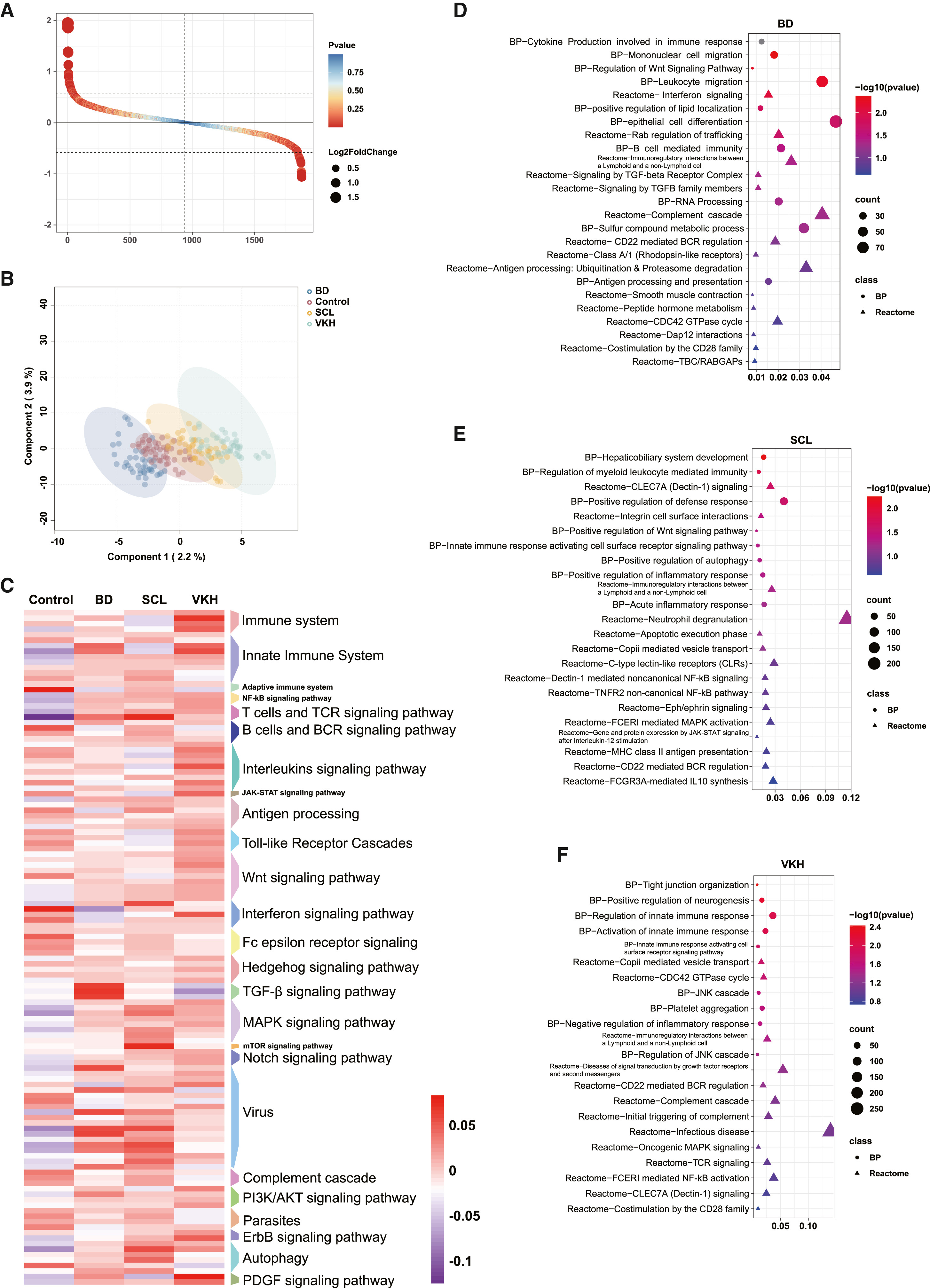
Li X R. et al. iScience. 2024.
MtoZ Biolabs, an integrated chromatography and mass spectrometry (MS) services provider.
Related Services
Peptide Biomarker Discovery & Validation Service
How to order?







