Ascitic Cell-derived Exosomes-based Vaccines
Ascitic Cell-derived Exosomes-based Vaccines development service helps customers build tumor-specific and immunogenic vaccine candidates based on clinical ascites samples for mechanism research, immune assessment and translational medicine applications.
Sujin Hyung. et al. Science Advances. 2023.
Exosomes are nanoscale double-membrane vesicles secreted by various cell types, carrying biological molecules such as proteins, lipids and nucleic acids from the source cells, and participating in intercellular signaling. Ascites, as a form of "liquid biopsy" in the development of malignant solid tumors, is rich in tumor cells, immune cells, and the cytokines and exosomes they release. Ascitic cell-derived exosomes (AEX), as exosomes secreted from ascites tumor cells, naturally carry key antigen information and immune regulatory molecules of the source cells, such as heat shock proteins (HSPs), adhesion molecules and co-stimulatory markers. These AEXs can present tumor antigens, activate dendritic cells (DCs) and T cells, and induce specific immune responses against tumors, thus playing an important role in cancer immunotherapy. AEX exhibits significant immune activation potential by carrying tumor-associated antigens and immune regulatory factors, and is particularly suitable for the development of personalized vaccines for advanced ascites tumors (such as colorectal cancer, ovarian cancer, etc.).
Leveraging advanced exosome isolation platforms and antigen profiling capabilities, MtoZ Biolabs provides Ascitic Cell-derived Exosomes-based Vaccines development service aimed at developing new cancer immunotherapy strategies using exosomes isolated from malignant ascites. The service covers the entire process from AEX separation and purification, property analysis, to vaccine design and functional verification, helping customers advance AEX-based vaccine research and application.
Analysis Workflow
MtoZ Biolabs provides one-stop Ascitic Cell-derived Exosomes-based Vaccines preparation and validation services, suitable for scientific research, preclinical validation and personalized treatment exploration:
1. Collection and processing of ascites samples
Samples were collected from the patient's ascites and centrifuged at low speed to remove cells and debris to obtain cell supernatant.
2. AEX separation and purification
Exosomes were isolated and purified from ascites supernatant using ultracentrifugation, density gradient centrifugation or size exclusion chromatography.
3. Characterization of AEX
The morphology, particle size distribution and surface markers of AEX were comprehensively analyzed using transmission electron microscopy (TEM), nanoparticle tracking analysis (NTA) and western blot to confirm its characteristics.
4. Vaccine preparation and functional verification
The purified AEX is combined with an immune adjuvant (such as GM-CSF) to prepare a vaccine preparation. Its ability to induce specific immune responses, including T cell proliferation, cytokine release, and antitumor activity, was evaluated in in vitro and in vivo models.
Applications
Personalized Cancer Vaccine Development
Utilize tumor antigens derived from individual patients' ascitic fluid to develop highly matched AEX-based vaccines.
Functional translational research on tumor liquid biopsy
Ascites exosomes are used for immune assessment, exploration of drug resistance mechanisms, and identification of immune markers.
Adjuvant Discovery
The abundance of HSPs and tumor antigens in AEX enables their use as natural immune adjuvants to enhance other vaccine platforms.
Oncofetal Antigen Immunotherapy Research
Use CEA-positive AEX to develop immune intervention strategies targeting CEA-expressing tumors such as colorectal and ovarian cancers.
FAQ
Q. Are Tumor-Specific Antigens Enriched in AEX? Will there be Contamination of Exosomes from Non-Tumor Sources?
AEX originates from tumor cells or tumor-associated immune cells in ascites, and naturally carries a variety of tumor-associated antigens (TAA) and carcinoembryonic antigens (such as CEA), and is also rich in immune active proteins such as HSP70 and MHC molecules. We verify its antigen spectrum through Western blot, ELISA, RNA-seq or proteomics, and can use markers such as CD63/CD81 to monitor the purity of exosomes, effectively distinguishing exosomes from tumor sources and non-specific sources to ensure functional targeting.
Q. Ascites Samples themselves Contain Inflammatory and Immunosuppressive Factors. Will this Affect the Effectiveness of the AEX Vaccine?
This is a critical consideration in AEX vaccine development. During exosome isolation, centrifugation and filtration steps are used to remove soluble cytokines and immunosuppressive factors like IL-10 and TGF-β. Further purification or co-delivery with dendritic cells can be implemented as needed to boost antigen presentation and immune activation, minimizing immunosuppressive effects.
Case Study
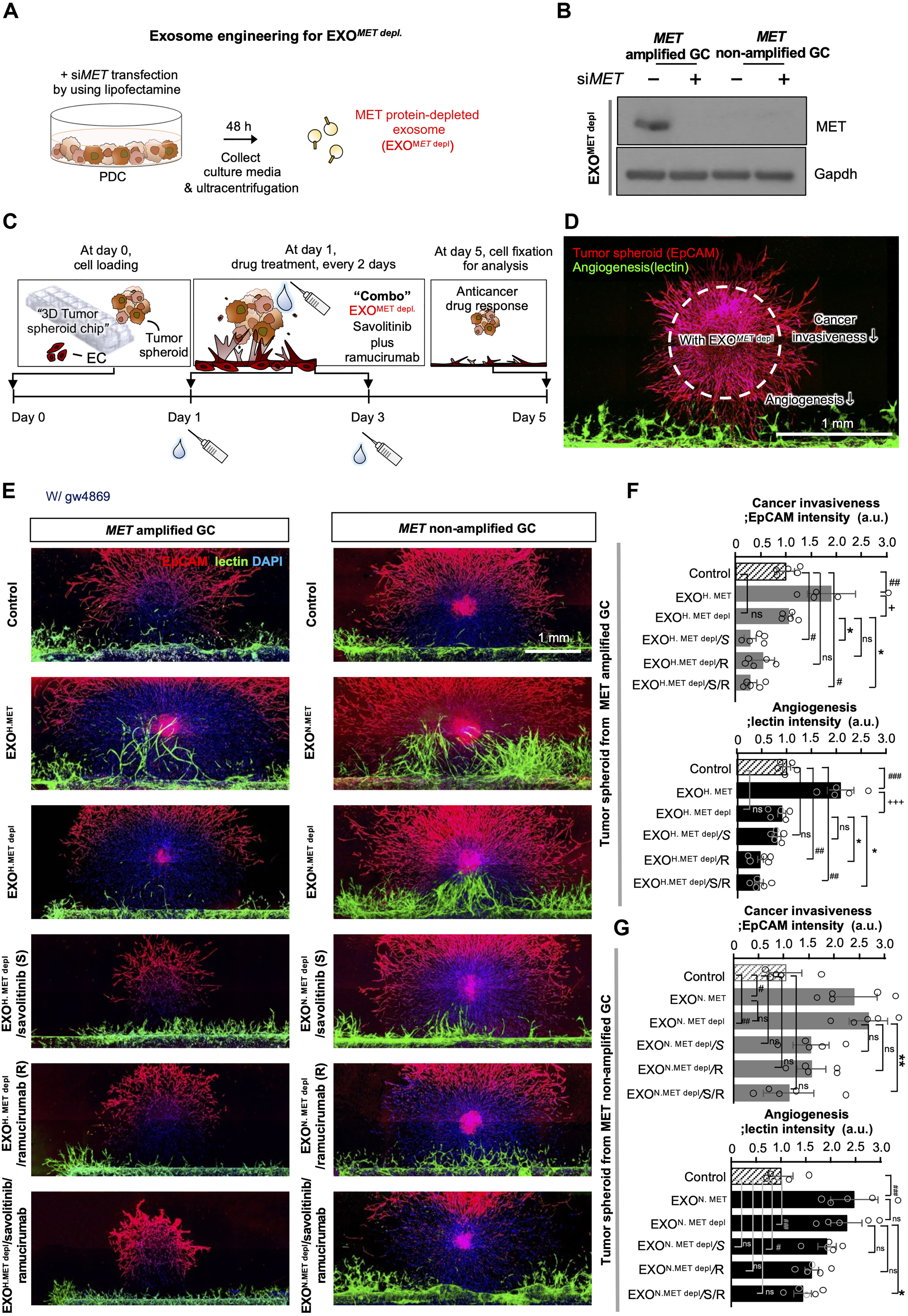
In this study, researchers isolated ascitic cell-derived exosomes (AEX) from late-stage gastric cancer patients using differential ultracentrifugation, followed by characterization via TEM, NTA, and Western blot. They confirmed that AEX from MET-amplified gastric cancer (GC) were enriched with the oncogenic MET protein. These AEX were preferentially taken up by gastric cancer cells, significantly promoting invasiveness and angiogenesis. In both patient-derived organoids and mouse models, removal of MET from AEX or combined treatment with MET/VEGFR2 inhibitors effectively reduced tumor activity. These findings demonstrate that AEX not only reflect tumor molecular status but also serve as both therapeutic targets and vaccine carriers—offering a promising strategy for personalized exosome-based therapies in ascitic tumors.
Sujin Hyung. et al. Science Advances. 2023.
How to order?







