Antibody Glycosylation Analysis Service
Antibody glycosylation analysis is a comprehensive analytical approach focused on detecting and characterizing the glycosylation features of antibody molecules. Antibody glycosylation refers to the covalent attachment of glycan chains to specific amino acid residues (such as Asn297 in the Fc region of IgG), which significantly impacts the antibody’s structural stability, biological activity, immune functions (e.g., ADCC, CDC), in vivo pharmacokinetics, and therapeutic efficacy. This service employs strategies such as enzymatic digestion, glycan release, or glycopeptide preservation, and integrates advanced technologies including high-resolution mass spectrometry (LC-MS/MS, MALDI-TOF), HILIC-UPLC, and capillary electrophoresis to systematically analyze glycan composition, linkage patterns, site distribution, and functional characteristics.
Antibody glycosylation analysis service is widely applied in monoclonal antibody development, biopharmaceutical consistency assessment, antibody quality control, process optimization, and glycoengineering. By providing detailed glycosylation profiling, this service facilitates the evaluation of structure–function relationships and supports the establishment of quality standards in antibody design and biologics development.
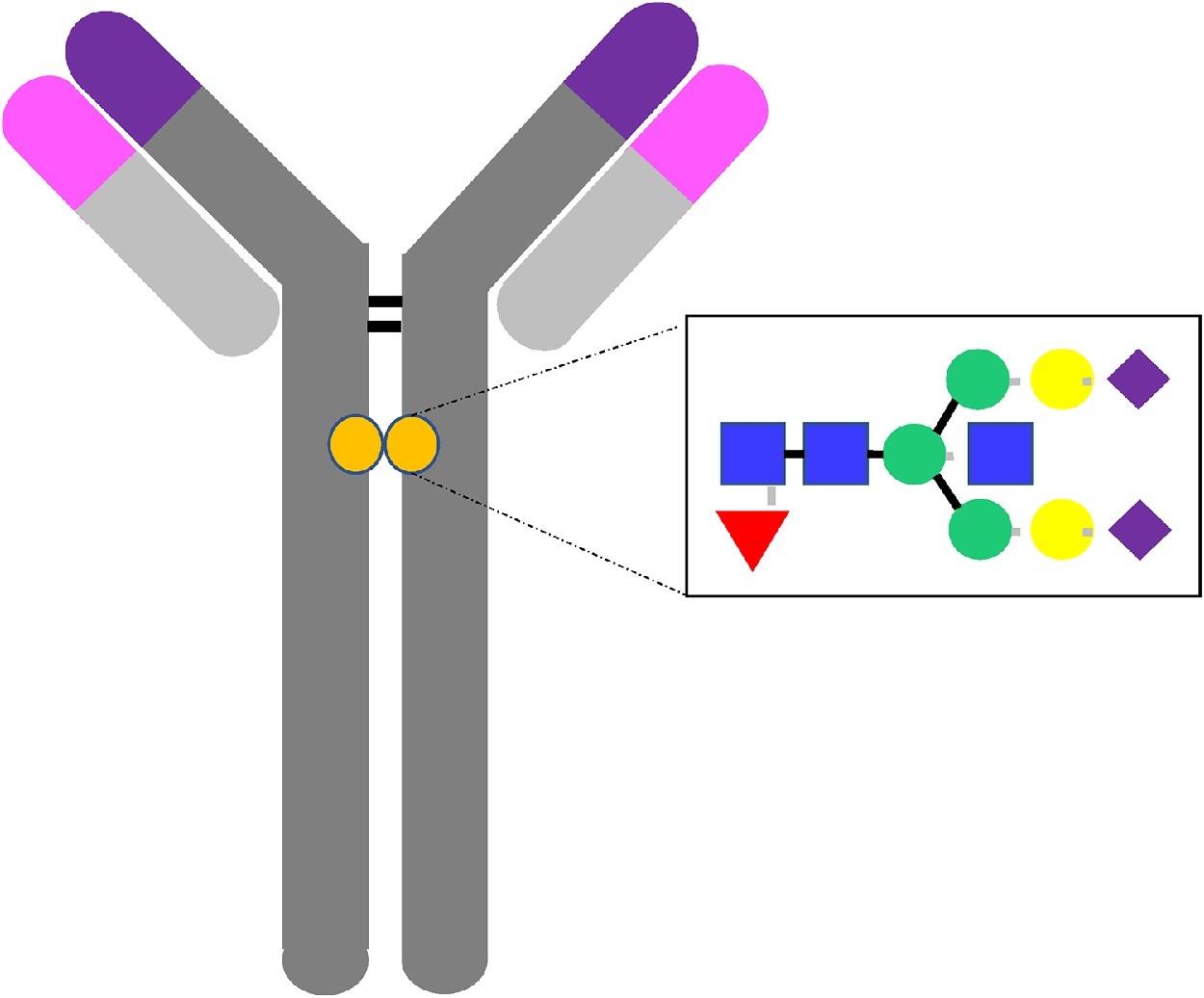
Alter, G. et al. Seminars in Immunology, 2018.
Figure 1. The Antibody and its Glycan.
Services at MtoZ Biolabs
Based on multiple advanced analytical platforms, MtoZ Biolabs provides Antibody Glycosylation Analysis Services to systematically characterize the glycan composition, glycan structures, and modification sites of antibodies. This service enables precise quantitative and structural analysis of N-glycosylation and O-glycosylation on specific antibodies such as IgG, IgA, and IgM. Through high-sensitivity mass spectrometry and expert data interpretation, MtoZ Biolabs delivers reliable analytical support for antibody drug development, quality control, and functional studies. The analytical methods commonly used for antibody glycosylation characterization include the following:
1. Mass Spectrometry (LC-MS/MS, MALDI-TOF)
Mass spectrometry is the most widely used approach for antibody glycosylation analysis. LC-MS/MS enables precise qualitative and quantitative analysis of glycopeptides or released glycans, identifying glycan composition, linkage patterns, and modification sites. MALDI-TOF is commonly used for rapid profiling of glycan mass distribution, making it suitable for preliminary screening. These methods offer high sensitivity and resolution, ideal for analyzing complex samples and low-abundance modifications.
2. High-Performance Liquid Chromatography (HILIC-UPLC, RP-HPLC)
HILIC-UPLC, often combined with fluorescently labeled glycans, is used to separate and quantify various glycoforms, allowing for comprehensive glycan distribution profiling. RP-HPLC is mainly employed for glycopeptide separation and is frequently coupled with mass spectrometry for structural confirmation. These techniques offer excellent stability and reproducibility, making them suitable for quality control and batch-to-batch consistency assessments.
3. Capillary Electrophoresis (CE)
Capillary electrophoresis separates glycan structural isomers based on their charge differences, offering high resolution and excellent reproducibility. It is particularly effective for detecting subtle glycoform variations and performing quantitative analysis, making it ideal for high-throughput comparison of antibody glycosylation profiles.
4. Glycan Microarray
Glycan microarray technology is used to study the binding specificity of antibodies toward various glycan structures and serves as a powerful tool for functional analysis. It enables evaluation of how glycosylation affects antibody interactions with receptors, FcγRs, or pathogen-associated carbohydrate antigens, and is widely applied in antibody functional validation and structure-guided optimization.
Analysis Workflow
1. Sample Preparation
Target antibodies are extracted from expression systems or purified samples, followed by reduction, alkylation, and enzymatic digestion. PNGase F may be used when N-glycan release is required.
2. Glycan or Glycopeptide Enrichment
Depending on the analytical objective, either released glycans or intact glycopeptides are preserved. Enrichment is performed using solid-phase extraction, HILIC, or affinity-based methods to enhance detection sensitivity.
3. Analytical Detection
Advanced platforms such as LC-MS/MS, MALDI-TOF, HILIC-UPLC, and CE are used for comprehensive qualitative and quantitative analysis of glycan structures, glycoform distribution, and modification sites.
4. Data Analysis and Reporting
Specialized software and glycan databases are used to interpret glycosylation patterns. Deliverables include glycan composition, site-specific modification information, abundance profiles, and functional annotations to support downstream antibody functionality and quality assessment.
Sample Submission Suggestions
1. Sample Types
Compatible with various antibody sources, including cell culture supernatants, purified monoclonal antibodies, and recombinant IgG. High-purity samples are recommended to ensure accurate glycosylation profiling.
2. Buffer Requirements
Avoid using buffers containing glycerol, SDS, EDTA, or high salt concentrations. Mass spectrometry–compatible buffers are recommended to prevent interference with glycan analysis.
3. Sample Transport
Samples should be stored at –80°C and shipped on dry ice. Repeated freeze–thaw cycles must be avoided to maintain glycosylation integrity. For liquid samples, lyophilized or concentrated forms are preferred.
Service Advantages
1. Diverse Technologies
Utilizes a combination of high-resolution mass spectrometry (LC-MS/MS, MALDI-TOF), HILIC-UPLC, and capillary electrophoresis to comprehensively analyze glycan structures, modification sites, and relative abundance—meeting various research depth requirements.
2. High-Sensitivity Detection
Optimized sample preparation and glycan enrichment workflows significantly improve the detection of low-abundance glycoforms, ensuring comprehensive glycosylation coverage and data reliability, even in complex samples.
3. Data Visualization
Delivers glycan structural maps, distribution charts, site-specific modifications, and quantitative data in a graphical report format, enabling intuitive interpretation and batch-to-batch comparisons for researchers.
4. Flexible Customization
Offers services ranging from standard profiling to advanced, customized solutions. Clients can tailor the workflow based on antibody type, research goals, and application needs, supporting drug development, process optimization, and mechanistic studies.
Applications
1. Antibody Drug Development and Optimization
Antibody glycosylation analysis service is used to evaluate the impact of different expression systems or glycoengineering modifications on antibody glycan structures, aiming to improve drug stability, immune activity, and clinical efficacy.
2. Structure–Function Relationship Studies
By analyzing how glycosylation affects antibody conformation, Fc receptor binding, and immune effector functions such as ADCC, this service supports in-depth exploration of structure–function mechanisms.
3. Disease Mechanism and Biomarker Discovery
Applicable for studying glycosylation pattern changes in disease-associated antibodies, facilitating the identification of potential biomarkers in autoimmune diseases, cancers, and related fields.
4. Vaccine and Immunotherapy Research
Antibody glycosylation analysis service can be used to assess the influence of glycosylation modifications on antibody-mediated immune responses, supporting vaccine development and the optimization of antibody-based immunotherapeutic strategies.
Case Study
1. High-Throughput Glycosylation Analysis of Intact Monoclonal Antibodies
This study aimed to establish a high-throughput glycosylation analysis method for intact antibodies to improve the efficiency and accuracy of glycoform profiling. The target samples were undigested monoclonal antibodies, and the method integrated capillary electrophoresis (CE), liquid chromatography (LC), and mass spectrometry (MS) for direct detection and quantification of N-glycans. By optimizing the sample buffer system and implementing online desalting strategies, the workflow demonstrated enhanced reproducibility and ease of operation. Results showed that the method could rapidly identify major glycoforms such as G0F, G1F, and G2F, as well as their variants, with high sensitivity, good resolution, and strong analytical throughput. The study concluded that the CE-LC-MS platform enables comprehensive glycosylation analysis of intact antibodies without enzymatic digestion or complex sample preparation, making it suitable for structural characterization and quality control in biopharmaceutical development.
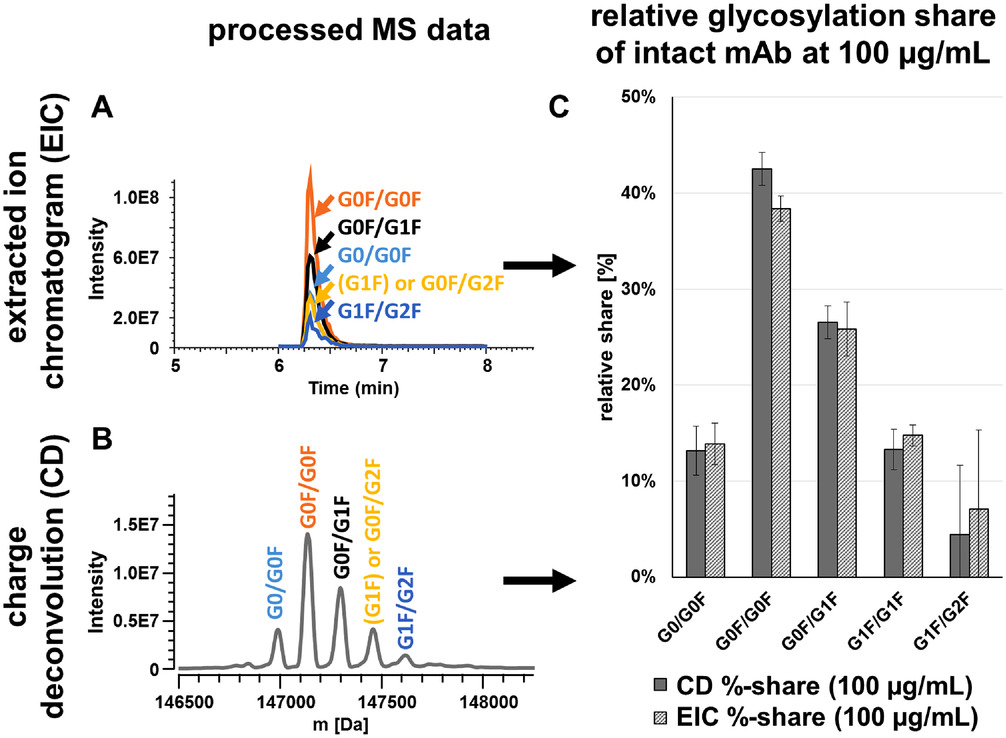
Naumann, L. et al. Journal of Separation Science, 2022.
Figure 2. Comparison of Ion Trace (A) and Spectral (B) Approach.
FAQ
Q1: What Types of Glycosylation Can this Service Detect?
A1: The service primarily detects N-glycosylation, particularly in the Fc region of IgG. Upon request, it can also analyze glycosylation in the Fab region or identify O-glycosylation sites. Various glycan types such as high-mannose, complex, and hybrid structures can be characterized.
Q2: Can Glycoform Profiles Be Compared Across Different Antibody Batches?
A2: Yes. We can compare glycan distributions across antibody samples from different production batches, cell lines, or expression conditions to assess batch-to-batch consistency or the effects of glycoengineering strategies.
How to order?







