Antibody-Drug Conjugate (ADC) Characterization Service
Antibody-drug conjugate (ADC) characterization aims to comprehensively analyze the critical quality attributes (CQAs) of ADC to ensure their structural integrity, uniformity, and biological activity. ADC is a targeted therapeutic agents composed of monoclonal antibody (mAb) conjugated to cytotoxic small-molecule drugs via controllable linkers. The core principle of ADC lies in leveraging the targeting ability of antibodies to deliver drugs, enhancing therapeutic efficacy while reducing systemic toxicity. Due to the complex structure of ADC, characterization analysis must encompass multiple aspects, including antibody sequence integrity, drug-to-antibody ratio (DAR), conjugation sites, linker stability, purity, and post-translational modifications (PTMs), to ensure the uniformity and safety of the drug.
Antibody-drug conjugate (ADC) characterization service is widely applied in biopharmaceutical development, antibody drug research, preclinical studies, and quality control. For instance, in drug development, this service can assess the impact of different conjugation strategies on ADC stability and biological activity, optimizing production processes to improve efficacy. During manufacturing, it can be used for batch-to-batch consistency analysis to ensure compliance with quality standards. Additionally, ADC characterization plays a crucial role in pharmacokinetics (PK) and in vivo metabolism studies, providing in-depth insights into ADC degradation and drug release mechanisms, thereby supporting clinical applications with scientific evidence.
Chen, T. et al. Journal of Chromatography B, 2016.
Figure 1. Depiction of a Typical ADC Comprised of a Monoclonal Antibody, Linker, and Small Molecule Drug.
Services at MtoZ Biolabs
Utilizing advanced analytical techniques such as high-resolution liquid chromatography-mass spectrometry (LC-MS/MS) and high-performance liquid chromatography (HPLC), MtoZ Biolabs provides comprehensive antibody-drug conjugate (ADC) characterization service to accurately analyze the critical quality attributes (CQAs) of ADC. Our services encompass antibody sequence identification, drug-to-antibody ratio (DAR) determination, conjugation site analysis, linker stability assessment, post-translational modification (PTM) detection, and purity analysis. By employing high-resolution mass spectrometry, ultraviolet-visible spectrophotometry (UV-Vis), isoelectric focusing electrophoresis (IEF), and other analytical methods, we ensure the structural integrity, consistency, and biological activity of ADC.
Service Advantages
1. High-Resolution Precision Analysis
MtoZ Biolabs leverages high-resolution mass spectrometry (LC-MS/MS) and high-performance liquid chromatography (HPLC) technologies to accurately determine the drug-to-antibody ratio (DAR), conjugation sites, post-translational modifications (PTMs), and purity, ensuring the structural integrity and functional stability of ADC.
2. One-Time-Charge
Our pricing is transparent, no hidden fees or additional costs.
3. High Sensitivity and High Reproducibility
Utilizing advanced protein analysis techniques, MtoZ Biolabs enhances the sensitivity for detecting low-abundance conjugates and minor modifications. Additionally, optimized data processing workflows ensure high reproducibility, providing reliable data support for ADC drug development.
4. Customized Analytical Solutions
MtoZ Biolabs offers flexible experimental designs tailored to different types of ADC, addressing research needs across various development stages, including early screening, process optimization, and preclinical evaluation.
Applications
1. ADC Drug Development and Quality Control
In the development of antibody-drug conjugate (ADC), antibody-drug conjugate (ADC) characterization service is essential for analyzing drug-to-antibody ratio (DAR), conjugation sites, linker stability, and purity. These analyses ensure structural homogeneity, enhance drug efficacy, and meet regulatory requirements.
2. Pharmacokinetics (PK) and Metabolism Studies
The in vivo metabolism and clearance mechanisms of ADC directly impact their therapeutic efficacy and safety. Characterization services facilitate the study of ADC degradation, active drug release, and biodistribution in different biological environments, optimizing drug design and improving clinical application potential.
3. ADC Process Optimization and Batch Consistency Evaluation
The conjugation efficiency, stability, and purity of ADC during production directly impact drug quality. Antibody-drug conjugate (ADC) characterization service enables quality comparison across different production batches, optimizing manufacturing processes and ensuring consistency and reproducibility in large-scale production.
4. ADC Safety and Toxicity Assessment
The conjugation patterns, release mechanisms, and metabolic pathways of different ADC drugs may affect their toxicity. High-resolution mass spectrometry and bioanalytical techniques are used to assess ADC stability and potential toxicity, providing scientific data for drug safety evaluation.
5. ADC Preclinical and Clinical Research
During the preclinical and clinical phases of ADC development, antibody-drug conjugate (ADC) characterization service supports studies on the in vivo behavior of antibodies and small-molecule drugs. These services ensure drug stability and efficacy in clinical trials, facilitating personalized treatment and precision medicine.
Case Study
1. Design and Characterization of Immune-Stimulating Imidazo[4,5-c]quinoline Antibody-Drug Conjugates
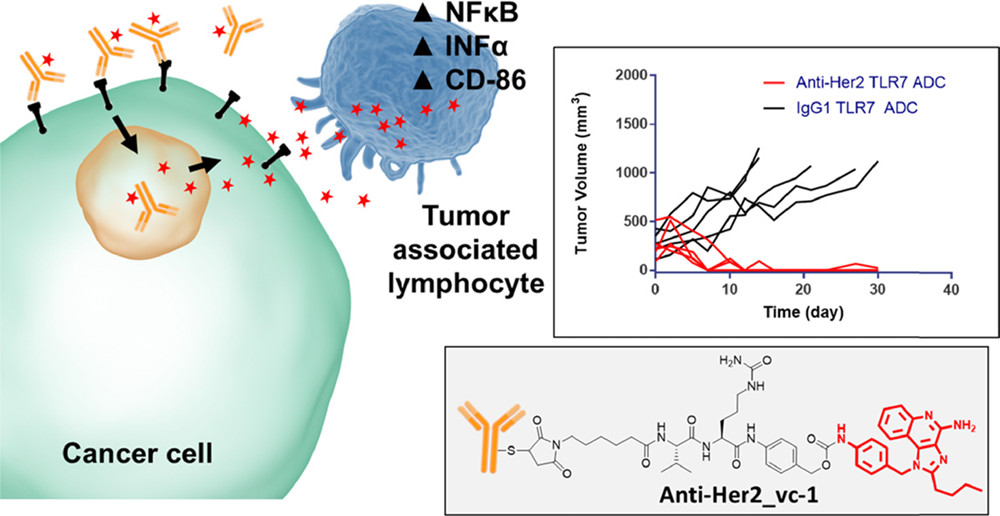
This study aimed to design and characterize immune-stimulating antibody-drug conjugate (ADC) based on imidazo[4,5-c]quinoline (IMQ), evaluating their physicochemical properties, stability, and biological activity. The study focused on IMQ-modified ADC molecules, utilizing high-resolution liquid chromatography-mass spectrometry (LC-HRMS) to analyze the drug-to-antibody ratio (DAR), purity, and drug release characteristics, while surface plasmon resonance (SPR) was employed to assess antigen-binding affinity. Differential scanning calorimetry (DSC) and dynamic light scattering (DLS) were used to evaluate thermal stability and aggregation behavior, while in vitro cell assays and in vivo mouse models were conducted to examine immune activation and antitumor efficacy. The results demonstrated that IMQ-ADC maintained antigen-binding affinity while exhibiting favorable physicochemical stability and effectively activating dendritic cells and T cells, thereby promoting cytokine secretion. Metabolic analysis indicated a controlled degradation pattern in vivo, ensuring stability and biological activity. The study concluded that comprehensive characterization confirmed the ADC’s favorable pharmacokinetic properties and immune-stimulating capabilities, providing essential experimental data for ADC drug design, optimization, and quality control.
Fang, S T. et al. Molecular Pharmaceutics, 2022.
Figure 2. The Overview of the Study.
Deliverables
1. Experiment Procedures
2. Parameters of Liquid Chromatography and Mass Spectrometer
3. MS Raw Data Files
4. ADC Evaluation Results
5. Bioinformatics Analysis
How to order?







