Anti target hERG Structure Determination Service | Cryo-EM
The human Ether-à-go-go-Related Gene (hERG) encodes a voltage-gated potassium channel that plays a pivotal role in cardiac repolarization. Unintended blockade of hERG channels by small-molecule drug candidates can delay ventricular repolarization, leading to prolonged QT intervals. QT prolongation is a recognized surrogate marker of proarrhythmic risk and is associated with potentially fatal cardiac events such as Torsades de Pointes. As a result, the hERG channel is widely regarded as a key anti-target in drug development.
Understanding the structural basis of drug–hERG interactions is essential for the rational design of compounds with minimized cardiotoxicity. However, the intrinsic complexity and conformational flexibility of membrane proteins such as hERG pose significant challenges for conventional structural biology approaches. Cryogenic electron microscopy (Cryo-EM) has emerged as a transformative tool in this context, enabling near-atomic resolution visualization of ion channels under near-native conditions.
High-resolution Cryo-EM analysis of hERG provides insight into its gating mechanisms, conformational states, and ligand-binding sites—information that is indispensable for structure-guided compound optimization and safety profiling. Structural determination of hERG is thus increasingly integrated into early-stage drug discovery workflows to proactively de-risk candidates and accelerate the development of safer, clinically viable therapeutics.
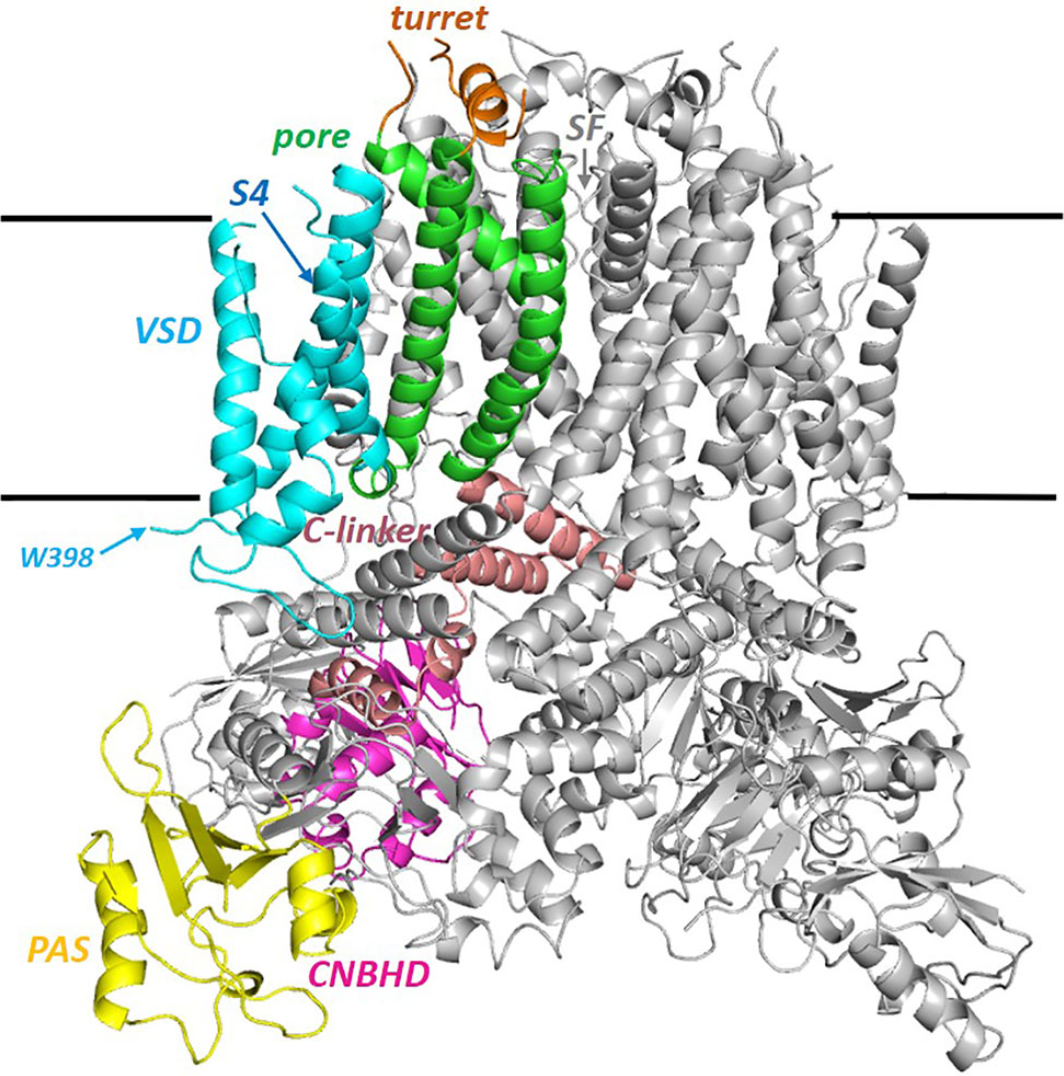
Figure 1. Side View of the Full hERG Cryo-EM Structure with One of the Four Subunits Colored by Domain
Service at MtoZ Biolabs
MtoZ Biolabs offers a specialized Anti target hERG Structure Determination Service based on high-resolution Cryo-EM. The Anti target hERG Structure Determination Service is tailored to support pharmaceutical researchers in understanding hERG channel architecture and its interactions with drug candidates, contributing to early cardiotoxicity risk assessment and safer compound design. Our Cryo-EM workflow enables detailed analysis of hERG channel conformation, ligand binding modes, and structural changes upon compound association. We support comparative evaluation of drug analogs, mapping of key interaction regions, and assessment of mutation-induced structural effects. All studies are performed by an experienced team using advanced instrumentation and standardized image processing pipelines. We deliver interpretable structural data and comprehensive reports that support both discovery-phase decision-making and regulatory submissions.
Analysis Workflow
1. Sample Preparation
Expression and purification of the hERG protein, either in full-length or domain-specific form, optionally in complex with test compounds or ligands.
2. Grid Preparation and Vitrification
Rapid freezing of purified protein samples under optimized buffer conditions using glow-discharged Cryo-EM grids to preserve native conformation.
3. Data Acquisition
High-resolution image collection using direct electron detectors under low-dose conditions, ensuring optimal signal-to-noise ratio.
4. Image Processing
Motion correction, particle picking, 2D classification, and 3D reconstruction to resolve the structural features of hERG and its ligand-bound states.
5. Structural Interpretation
Model building and density fitting to localize binding sites, assess conformational states, and compare structural effects of different compounds or mutations.
6. Reporting and Deliverables
Delivery of a comprehensive report, including representative micrographs, tailored structural interpretation, and other detailed information.
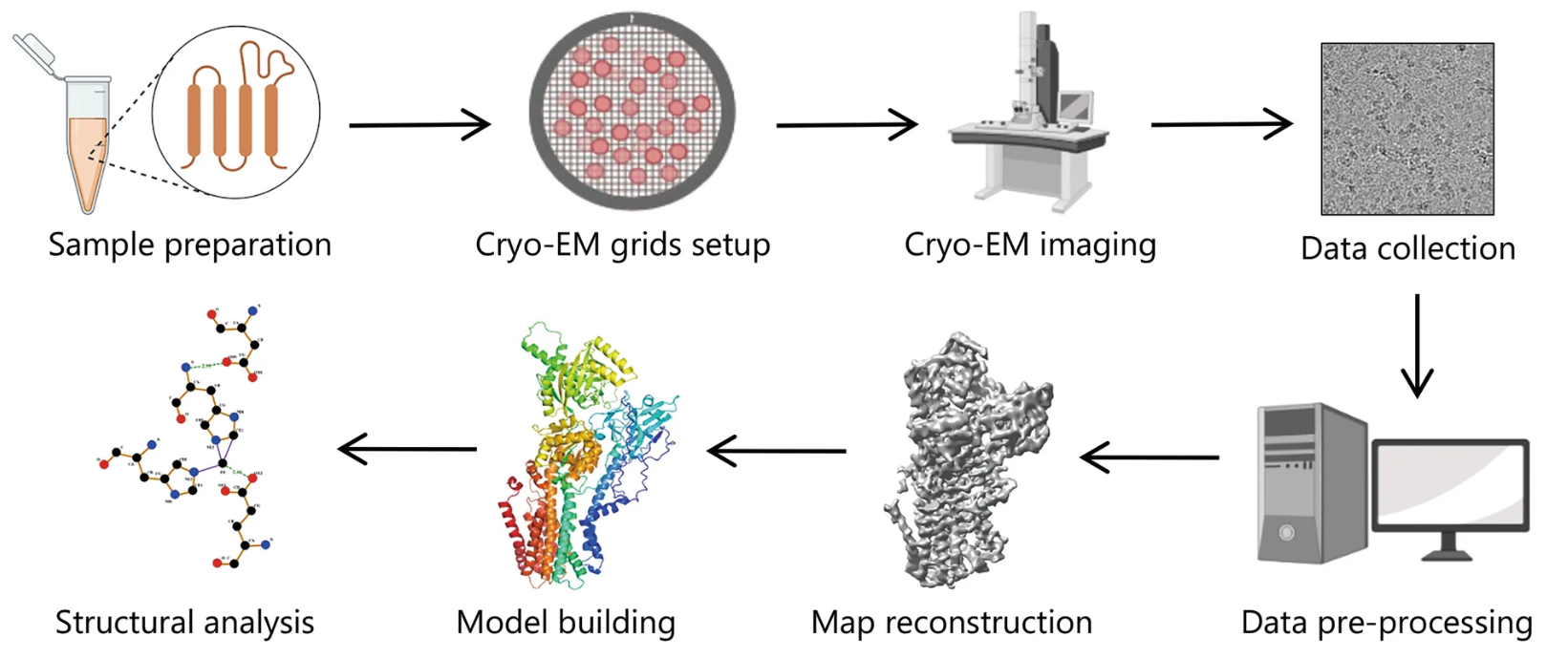
Figure 2. Typical Workflow of Single Particle Cryo-EM for Structural Analysis
Service Advantages
1. Tailored Project Execution: We offer flexible project scopes to accommodate early discovery screens, structural comparisons, or regulatory-supporting studies, with full transparency in execution timelines and deliverables.
2. Collaborative Scientific Support: Our scientific team works closely with clients to define objectives, troubleshoot technical challenges, and ensure that delivered data meet both research and regulatory needs.
3. Efficient and Confidential Workflow: Projects are managed with a focus on efficiency, quality, and confidentiality—ensuring rapid progress without compromising data integrity or intellectual property protection.
Applications
1. Mechanistic Studies
Cryo-EM enables direct visualization of hERG conformational states and gating transitions, facilitating detailed investigations into the structural mechanisms of ion conduction, inactivation, and drug-induced inhibition.
2. Drug Design and Optimization
Structural data on compound–hERG interactions support rational design of molecules with reduced off-target activity. This allows medicinal chemists to optimize selectivity and minimize cardiotoxicity risk during lead refinement.
3. Cardiac Safety Evaluation
Our service helps assess whether drug candidates bind to or alter the structure of the hERG channel, contributing to early-stage cardiotoxicity screening and supporting go/no-go decisions in preclinical pipelines.
4. Regulatory Support
High-resolution structural insights into hERG binding contribute to mechanistic justifications in regulatory filings.
Case Study
Structural Insights into hERG Blockade by Astemizole via Cryo-EM
A recent cryo-electron microscopy (Cryo-EM) study resolved the structure of the human hERG potassium channel in complex with potassium ions and the well-known inhibitor astemizole at 3.5 Å resolution. As a drug associated with QT prolongation and proarrhythmic risk, astemizole served as a model compound for analyzing hERG off-target binding. The study revealed that astemizole binds directly beneath the selectivity filter of the hERG channel, physically obstructing potassium conduction. This binding mode provides structural insight into the molecular mechanism of hERG inhibition and helps explain the channel’s sensitivity to certain drug scaffolds. This case exemplifies the value of anti target hERG structure determination using Cryo-EM, providing direct structural evidence of drug–channel interactions to inform cardiotoxicity risk assessment in drug development.
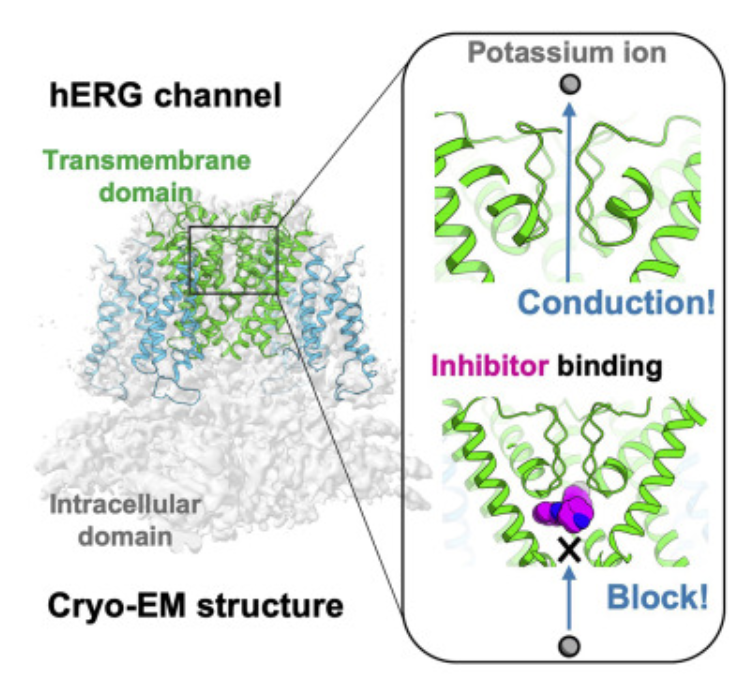
Asai, T. et al. Structure. 2021.
FAQ
Q: What is the goal of determining the hERG structure in drug development?
The structural determination of hERG enables direct visualization of drug-binding modes and conformational changes, providing critical insights for minimizing cardiotoxicity risk during lead optimization.
By partnering with MtoZ Biolabs, researchers can gain critical insights into hERG channel interactions, facilitating the development of safer and more effective therapeutics. For more information or to discuss your project needs, please contact us.
How to order?







