Allograft Rejection Detection-Applied Exosomes in Organ Transplantation
Allograft Rejection Detection-Applied Exosomes in Organ Transplantation monitors the immune status after organ transplantation and identifies the occurrence of acute or chronic rejection reactions by detecting exosomes and their biomolecular composition in the recipient's body fluids (such as blood and urine) to achieve early warning and intervention.
Organ transplantation is an important clinical means to treat end-stage organ failure, but transplant rejection (Allograft Rejection) is still the main challenge affecting the success rate and long-term survival rate of transplantation. In order to improve the long-term survival rate of transplanted organs, efficient and accurate transplant rejection detection (Allograft Rejection Detection) is crucial. Traditional transplant rejection detection methods include blood tests, tissue biopsies, imaging evaluations, etc., which have problems such as strong invasiveness, delayed detection, and limited sensitivity. Therefore, finding non-invasive and highly biologically specific detection methods has become the focus of current research.
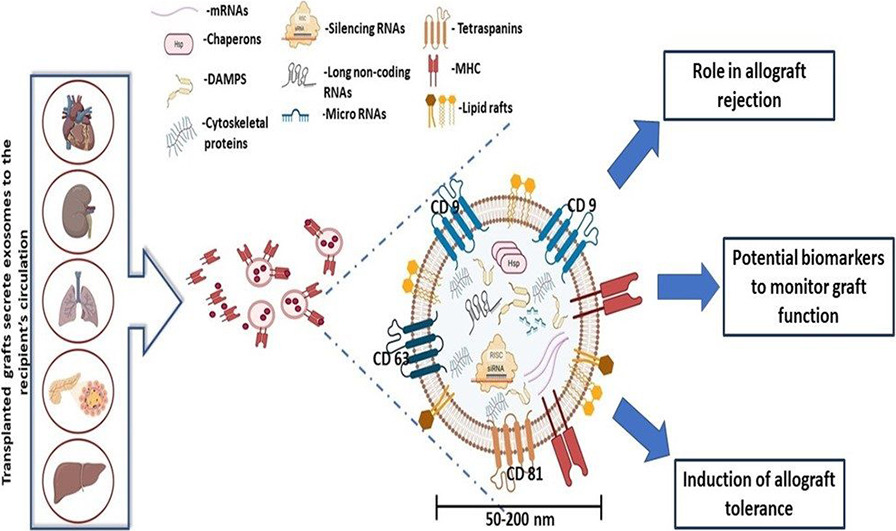
Saravanan P B. et al. Life Sciences. 2023.
Exosomes are small extracellular vesicles that play a crucial role in intercellular communication. During allograft rejection, donor cells, immune cells, and endothelial cells release specific exosomes. These exosomes carry proteins, miRNAs, mRNAs, and metabolites, whose dynamic changes during the rejection process can serve as potential non-invasive biomarkers to reflect the immune status of the transplanted organ.
Leveraging advanced multi-omics analysis platforms, MtoZ Biolabs provides comprehensive exosome isolation and analysis solutions for Allograft Rejection Detection-Applied Exosomes in Organ Transplantation. Our services aim to identify exosome-related biomarkers that meet the needs of rejection diagnosis and risk prediction research, facilitating early detection of rejection events and guiding personalized immunotherapy.
Advantages of Exosome-Based Allograft Rejection Detection
Non-Invasive Detection: Exosomes can be extracted from blood and urine, eliminating the need for invasive tissue biopsies.
High Sensitivity and Specificity: Exosome-based detection enables the highly sensitive and specific identification of subtle changes in graft status.
Real-Time Dynamic Monitoring: Suitable for long-term follow-up, allowing for early prediction of rejection events.
Personalized Treatment: Can be used to evaluate the efficacy of immunosuppressive therapy, supporting individualized treatment strategies.
Analysis Workflow
1. Exosome Isolation and Quality Control
High-purity exosomes are isolated using multiple techniques and undergo rigorous quality assessment.
2. Extraction of Exosomal Proteins or RNA
Target biomolecules are extracted from exosomes based on project requirements.
3. Biomarker Detection
Proteomics, metabolomics, or high-throughput sequencing technologies are used to analyze the extracted biomolecules.
4. Data Analysis and Report
Comprehensive data processing and bioinformatics analysis are conducted, with a detailed report provided.
FAQ
Q. How is Exosome Purity and Quality Validated to Ensure Suitability for Allograft Rejection Research?
Quality control (QC) of exosomes is essential to ensure their purity and integrity for transplantation rejection studies. The primary validation methods include:
1. Morphological Verification: Transmission electron microscopy (TEM) is used to observe the characteristic cup-shaped structure of exosomes, ensuring they remain intact and undamaged.
2. Size and Concentration Measurement: Nanoparticle tracking analysis (NTA) is performed to determine the size distribution and concentration of exosomes, excluding contamination from large vesicles or microparticles.
3. Protein Marker Detection: Western blot or ELISA is used to confirm the presence of exosome-specific markers such as CD9, CD63, and CD81, while simultaneously ruling out cytoplasmic contamination. HLA molecule detection can further evaluate exosomes' role in transplantation immunity.
4. Flow Cytometry (Exo-FACS): Single-particle exosome detection using fluorescently labeled flow cytometry improves specificity and enables precise exosome characterization.
By integrating these QC techniques, high-purity and high-stability exosomes can be ensured, providing reliable samples for allograft rejection research.
Case Study
This study isolated exosomes from the plasma of four heart transplant recipients and used magnetic beads conjugated with anti-donor HLA I antibodies to enrich donor heart-derived exosomes. C4d protein expression was then analyzed as a biomarker for acute versus antibody-mediated rejection (AMR) following heart transplantation. The results showed that in the three patients without rejection, troponin protein and mRNA were consistently expressed in their circulating donor heart-derived exosomes at all follow-up time points. One patient was diagnosed with AMR via endomyocardial biopsy on post-transplant day 14. After treatment, C4d protein expression in donor heart-derived exosomes decreased.
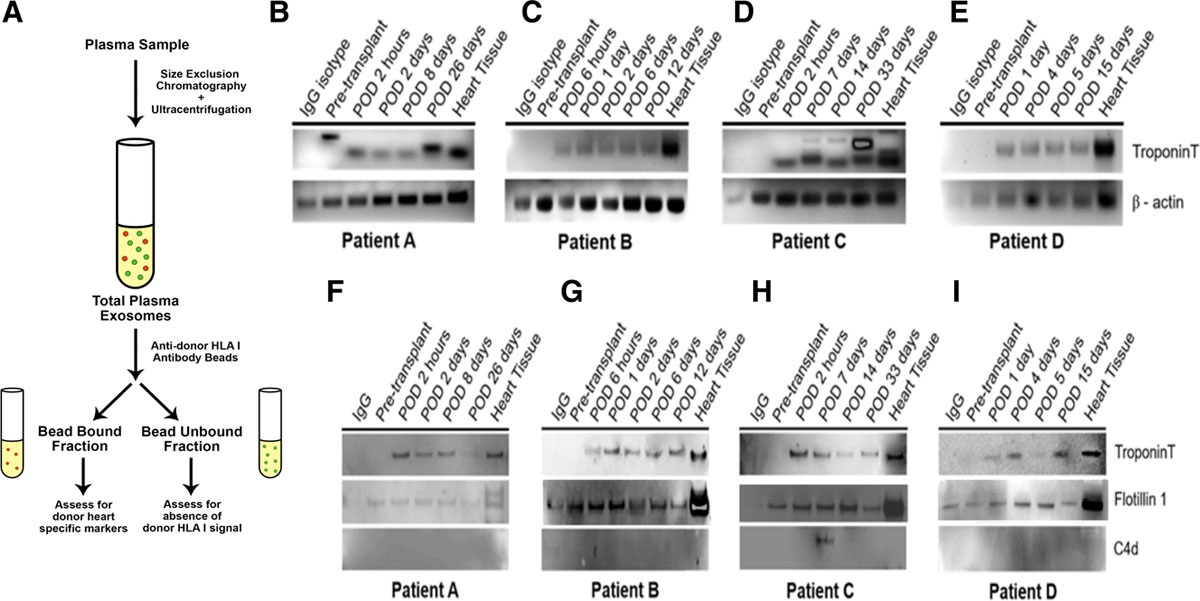
Hu RW. et al. Transplantation Direct. 2020.
How to order?







