Adeno-Associated Virus (AAV) Structure Characterization Service | Cryo-EM
Adeno-associated virus (AAV) is a non-pathogenic single-stranded DNA virus that has been widely applied in gene therapy, vaccine delivery, and cell therapy due to its high gene transfer efficiency and favorable safety profile. Among related technologies, adeno-associated virus (AAV) structure characterization is a high-resolution structural analysis service based on cryogenic electron microscopy (Cryo-EM), specifically designed to examine the three-dimensional morphology, capsid structure, empty-to-full particle ratio, and assembly integrity of AAV particles. This service involves rapid vitrification of AAV samples at liquid nitrogen temperature and imaging under low-dose, stain-free conditions, allowing for faithful reconstruction of native structures with nanometer-level resolution.
Adeno-associated virus (AAV) structure characterization service is widely used in the development of gene therapy vectors, vaccine design, viral capsid engineering, and quality control of biopharmaceuticals. Cryo-EM enables researchers to gain in-depth insights into AAV structural stability, packaging efficiency, and capsid variability, providing essential structural evidence and decision-making support for optimizing AAV design, validating vector function, and improving manufacturing processes.
Services at MtoZ Biolabs
Based on the state-of-the-art cryogenic electron microscopy platform, the adeno-associated virus (AAV) structure characterization service based on Cryo-EM provided by MtoZ Biolabs enables rapid freezing and in situ fixation of AAV samples under ultra-low temperature conditions, achieving high-resolution imaging without staining and with low-dose electron exposure. This service delivers critical data including the three-dimensional structure of AAV, capsid architecture, empty-to-full particle ratio, and assembly integrity. Through automated image acquisition and structural reconstruction, the results ensure nanometer-scale spatial resolution and high structural fidelity. It is ideally suited for various applications such as AAV vector quality control, structural optimization, and process validation.
Analysis Workflow
1. Sample Freezing Fixation
AAV samples are rapidly cooled to liquid nitrogen temperature to form vitreous ice, preserving the native structure of viral particles.
2. Cryo-EM Image Acquisition
Low-dose, high-resolution imaging is performed using a cryogenic transmission electron microscope to prevent radiation damage and ensure image quality.
3. Image Preprocessing and Particle Identification
Raw images are denoised, aligned, and screened to extract high-quality viral particle data for downstream analysis.
4. 3D Reconstruction and Structural Analysis
Computational methods are used to reconstruct the AAV capsid’s 3D structure, enabling analysis of empty-to-full particle ratios, capsid symmetry, and structural stability.
5. Data Delivery and Reporting
Original images, 3D models, and a detailed professional analysis report are provided to support AAV vector development, manufacturing optimization, and quality control.
Service Advantages
1. Real Structure Restoration
AAV samples are rapidly vitrified without staining agents, maintaining their native state and preventing structural interference or distortion for true-to-nature visualization.
2. High Resolution
Enabled by advanced Cryo-EM platforms, the service achieves nanometer-scale imaging to precisely resolve AAV capsid symmetry, subunit details, and nucleic acid content.
3. Distinction Between Full and Empty Particles
Allows quantitative assessment of the ratio between empty and genome-filled capsids, supporting packaging efficiency evaluation and critical quality release testing.
4. Broad Serotype Compatibility
Applicable to a wide range of natural and engineered AAV serotypes, including tissue-specific variants and self-complementary AAVs.
5. Supports R&D and Quality Control
Ideal for AAV vector design, batch-to-batch consistency assessment, manufacturing process optimization, and regulatory-compliant quality control in gene therapy development.
Applications
1. Gene Therapy Vector Development
Adeno-associated virus (AAV) structure characterization service enables detailed analysis of capsid structures and empty/full particle states across various serotypes and engineered variants, guiding vector design and candidate selection.
2. Packaging Efficiency Evaluation
By accurately distinguishing empty from full capsids, this service supports assessment of genome packaging quality and informs optimization of production processes.
3. Process Development and Consistency Verification
Comparative structural analysis across different manufacturing batches helps refine production methods and ensure product consistency and stability.
4. Structure–Function Mechanism Studies
Adeno-associated virus (AAV) structure characterization service reveals structural features related to receptor binding, tissue tropism, and immune evasion, providing key insights for both fundamental research and applied development.
Case Study
1. Structural Characterization of an Envelope-associated Adeno-Associated Virus Type 2 Capsid
This study aimed to characterize the capsid structure of envelope-associated adeno-associated virus type 2 (EA-AAV2) using cryo-electron microscopy (Cryo-EM) to evaluate whether the presence of a membrane alters its structural features. The research focused on EA-AAV2 particles produced via Sf9 cell expression and employed Cryo-EM for three-dimensional reconstruction. The results showed that the capsid structure of EA-AAV2 is largely consistent with that of conventional non-enveloped AAV2, with no significant differences in symmetry or surface morphology, indicating that the membrane does not affect capsid conformation. Cryo-EM also revealed a complete membrane envelope, suggesting potential advantages in in vivo stability and immune evasion. The study demonstrates that Cryo-EM is an effective tool for accurately characterizing the structure of AAV-based viruses and supports the development and application of novel AAV vectors.
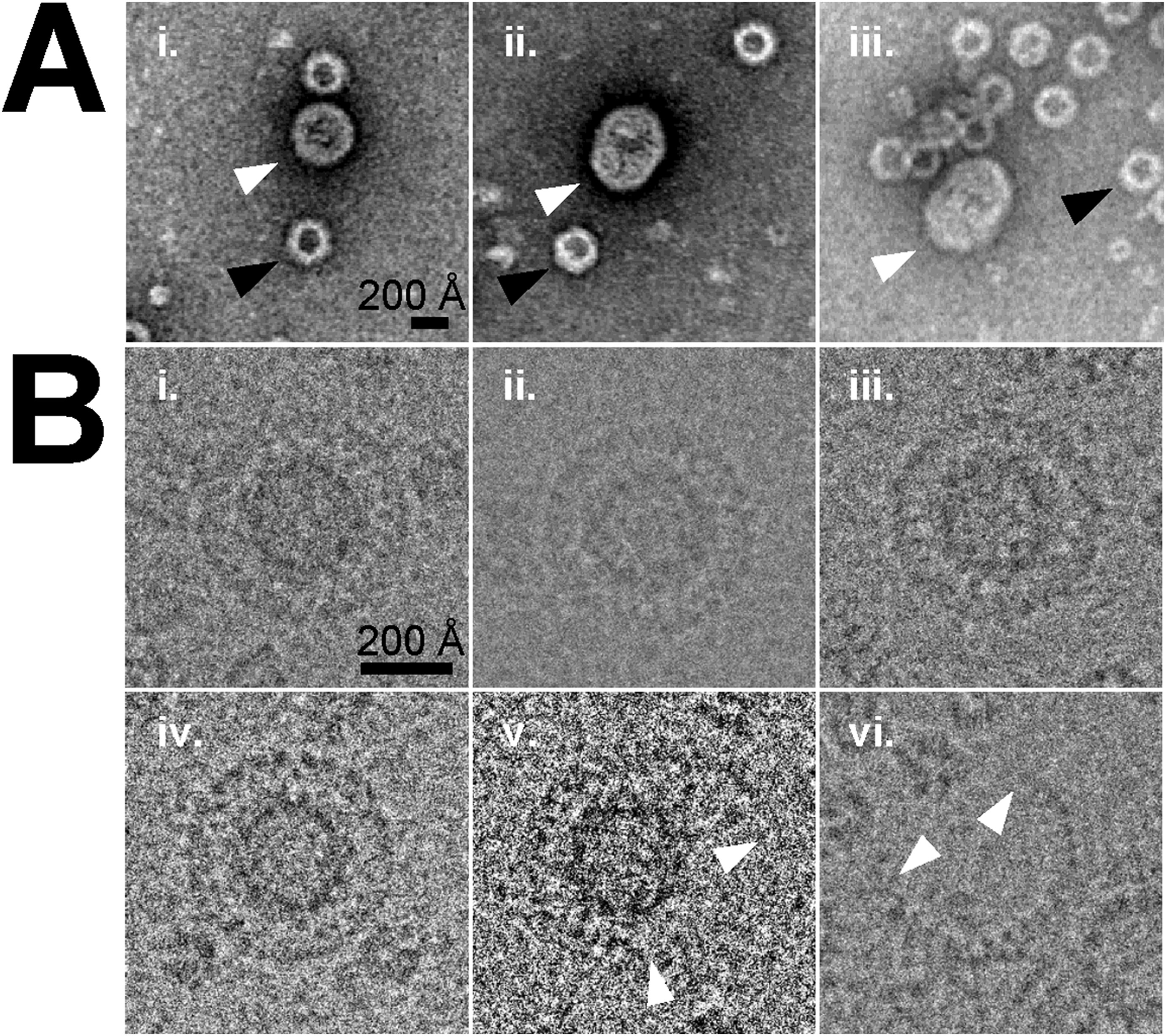
Hull, J A. et al. Virology, 2022.
Figure 1. Enveloped Particle Identification.
FAQ
Q1: Why Is Cryo-EM Suitable for AAV Structural Analysis?
A1: Cryo-EM enables in situ imaging without damaging the structure of AAV particles. It requires no staining, thus avoiding sample distortion, and allows for accurate visualization of the viral capsid and internal nucleic acid architecture. This makes it especially well-suited for finely structured and environmentally sensitive viral vectors.
Q2: Can this Service Distinguish Between Empty and Full AAV Particles?
A2: Yes. By analyzing image density differences and performing 3D reconstruction, Cryo-EM can effectively differentiate between empty capsids and genome-filled particles. It also provides quantification of their ratio, supporting accurate evaluation of packaging efficiency.
How to order?







