Glycan Profiling Service
Glycosylation is an important post-translational modification (PTM) of proteins. Based on the different linkage modes between the glycan and peptide chains, protein glycosylation can be divided into N-glycosylation and O-glycosylation. N-glycosylation involves the linkage between the reducing end of N-acetylglucosamine (GlcNAc) and the nitrogen atoms on certain asparagine (Asn) side chain amides in the peptide chain. The Asn that can carry a glycan must be in the motif of Asn-X-Ser/Thr 3, where X can be any amino acid residue except proline. O-glycosylation has a simpler structure than N-glycosylation. It generally has shorter glycan chains, but it is much more varied. The main residues in peptide chains that can be glycosylated are serine (Ser) and threonine (Thr), and also tyrosine, hydroxylysine, and hydroxyproline, with the linkage site being the hydroxyl oxygen atoms on these residue side chains. MtoZ Biolabs uses the high-resolution mass spectrometer Orbitrap Fusion Lumos and Byonic software, which includes nearly all types of glycans, to perform glycan analysis on peptides, proteins, and antibody drugs, determining glycosylation sites, types of N-glycans/O-glycans, and glycan compositions at the glycosylation sites.
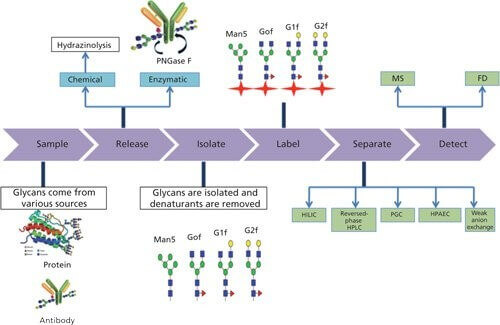
Gunjan, Narula. et al. LC-GC Europe. 2016.
Figure 1. Glycan Profiling and Classification
Deliverables
1. Experimental Procedures
2. Relevant Mass Spectrometry Parameters
3. Mass Spectrometry Images
4. Raw Data
5. Glycan Profiling Results
How to order?







