Succinylation Proteomics Analysis Service
- Analyzing the regulatory role of succinylation modifications in metabolic reprogramming in tumor cells.
- Investigating the link between mitochondrial protein aberrant modifications and oxidative stress in neurodegenerative diseases.
- Screening pathogen-specific modifying enzymes as novel antibacterial targets.
- Evaluating the regulatory effects of small-molecule inhibitors on modifying enzyme activity.
- Analyzing the modification dynamics of key enzymes in crops under drought stress.
- Constructing a modification regulation map for plant energy metabolism networks.
Succinylation proteomics analysis is a systematic research approach based on high-resolution mass spectrometry, aimed at comprehensively analyzing the distribution of succinylation modification sites, their dynamic regulation, and biological functions in proteins. As a lysine post-translational modification that has gained attention in recent years, succinylation plays a crucial role in cellular metabolic regulation, signal transduction, and disease progression by altering protein charge, spatial conformation, and interaction networks.
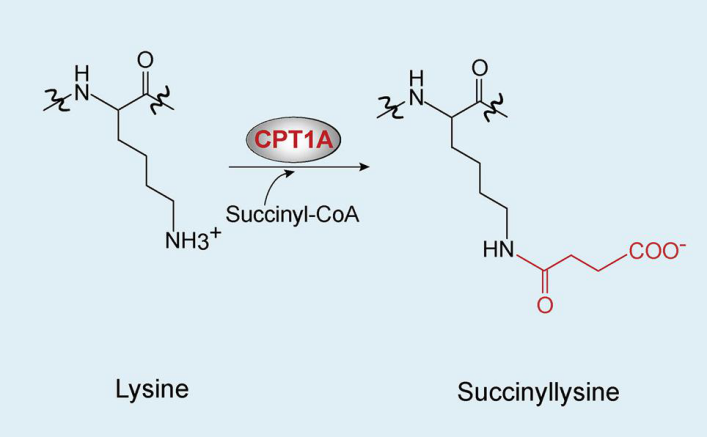
Kurmi, K. et al. Cell Rep. 2018.
Figure 1. Lysine Succinylation
Recently, succinylation has attracted considerable attention in fields such as mitochondrial metabolism, energy conversion, tumor biology, and immune regulation. However, due to its dynamic nature and low abundance, traditional research methods face challenges in precisely analyzing its regulatory mechanisms. To overcome these challenges in dynamic tracking and site identification accuracy, MtoZ Biolabs offers comprehensive Succinylation Proteomics Analysis Service using the advanced Orbitrap Fusion Lumos mass spectrometry platform and optimized enrichment strategies. Our Succinylation Proteomics Analysis Service is widely applied in cancer research, metabolic disease target screening, and antimicrobial resistance studies, helping researchers break through the bottlenecks in dynamic protein modification research.
Services at MtoZ Biolabs
The in-depth analysis of succinylation proteomics relies on the integration of multiple technologies. MtoZ Biolabs utilizes a combined strategy of chemical derivatization and immunoprecipitation, employing succinylation-specific antibodies for peptide enrichment, coupled with stable isotope labeling (TMT/iTRAQ) for quantitative analysis.
Using high-precision mass spectrometry fragmentation patterns (HCD/ETD), we can accurately differentiate succinylation modifications from other lysine modifications, such as acetylation and ubiquitination. Additionally, machine learning algorithms are employed to perform secondary verification of fragment ions, ensuring the specificity of site identification. For low-abundance modified peptides, we have developed a nano-liquid chromatography gradient optimization scheme, increasing the detection sensitivity to the fmol level.
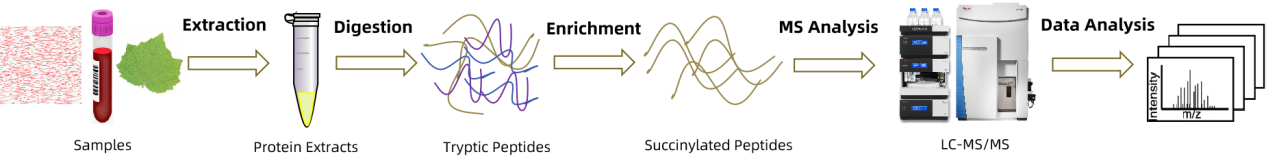
Analysis Workflow
To ensure the accuracy and reproducibility of the data, the Succinylation Proteomics Analysis Service adopts an optimized proteomics analysis process, which covers comprehensive services from sample preparation to data interpretation.
1. Sample Preparation: Optimization of protein extraction and digestion strategies to obtain high-quality peptides, improving detection efficiency.
2. Succinylation Peptide Enrichment: Utilization of specific antibody-based affinity enrichment or chemical labeling enrichment strategies to enhance the detection sensitivity of low-abundance modifications.
3. Liquid Chromatography Separation: Use of high-performance liquid chromatography (HPLC) or ultra-high-performance liquid chromatography (UPLC) to separate complex peptide mixtures, reducing background interference and improving analytical accuracy.
4. High-Resolution Mass Spectrometry Detection: Combining Orbitrap or Q-TOF mass spectrometry systems to accurately measure the m/z ratios and modification sites of succinylation.
5. Bioinformatics Analysis: Using specialized databases for succinylation site identification, quantitative analysis, and pathway enrichment analysis to investigate the role of succinylation in cellular functions and diseases.
Why Choose MtoZ Biolabs?
MtoZ Biolabs’ Succinylation Proteomics Analysis Service offers four key advantages:
1. Ultra-High Sensitivity: Capable of detecting low-abundance succinylation-modified proteins in samples.
2. Precise Quantitative Analysis: Application of labeling methods such as TMT/iTRAQ to achieve high-precision quantitative analysis of succinylation modifications.
3. Multi-Omics Integration: Supports integrated analysis with other modification omics, such as phosphorylation and acetylation, revealing cross-regulation mechanisms.
4. Functional Verification Support: Provides downstream verification services such as point mutation vector construction and molecular dynamics simulations.
Sample Submission Suggestions
To obtain high-quality succinylation proteomics data, MtoZ Biolabs has specific requirements regarding sample type, quality, and storage conditions. Common acceptable sample types include:
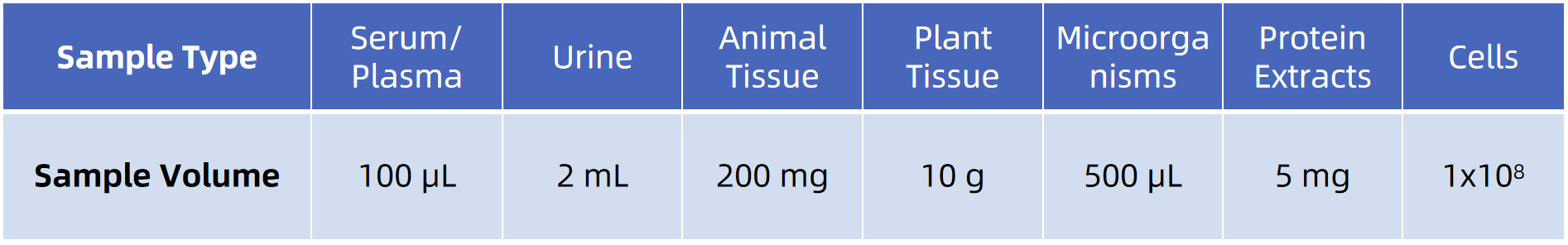
Sample preservation conditions, transportation methods, and buffer requirements are critical to experimental quality. MtoZ Biolabs provides detailed sample submission guidelines to ensure your samples meet the optimal analysis conditions. For special sample types or preparation requirements, please feel free to contact our technical support team to optimize your experimental plan.
Applications
· Disease Mechanism Research
· Drug Target Development
· Plant Stress Resistance Research
Case Study
Case 1: Revealing Protein Interaction Modifications with Succinylation Proteomics Analysis
In this study, mass spectrometry (LC-MS/MS) was used to identify a large number of lysine acetylation sites in Hydrophilus bacterium, revealing the complex interplay between acetylation and succinylation. By analyzing 3,189 lysine acetylation sites in 1,013 proteins, the study demonstrated the significant role these modifications play in bacterial metabolic pathways. Notably, the acetylation and succinylation at the K165 site of LuxS protein showed a cross-interaction, significantly affecting bacterial quorum sensing and enzyme activity.
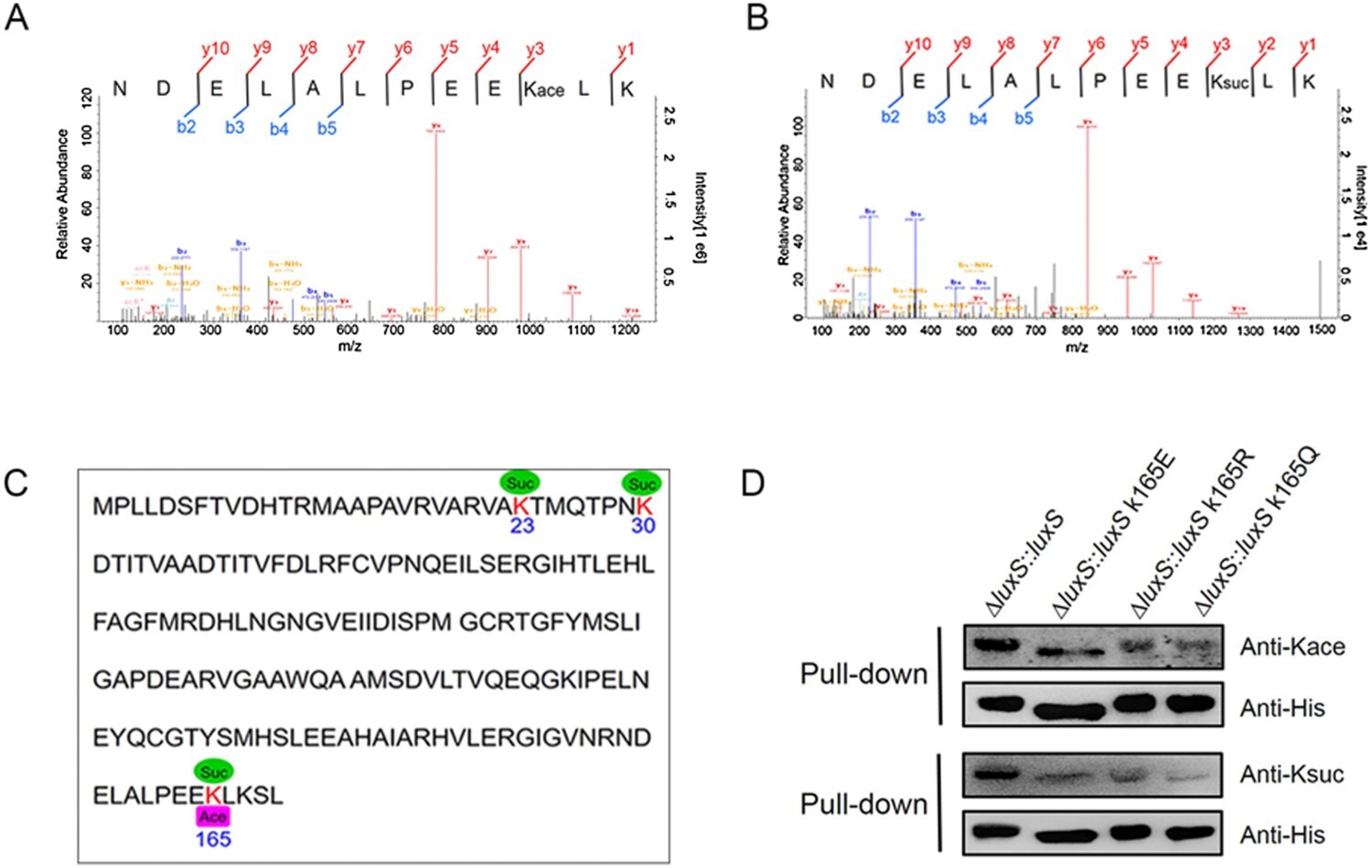
Sun, L. et al. Emerg. Microbes Infect. 2019.
Case 2: Succinylation Proteomics Analysis Reveals Tumor Angiogenesis Mechanism
Through TMT labeling and high-resolution LC-MS/MS, this study analyzed succinylation differences between glioma and normal endothelial cells, highlighting the high succinylation at K40 of TAGLN2. This modification significantly promoted glioma angiogenesis and tumor growth. Furthermore, overexpression of TAGLN2K40E exacerbated tumor cell proliferation and migration, while blocking this succinylation site reduced these effects. The study revealed the crucial role of TAGLN2 K40 succinylation in glioma development and its potential as a therapeutic target. This case study emphasizes the application value of the "Succinylation Proteomics Analysis Service" in exploring tumor biology.
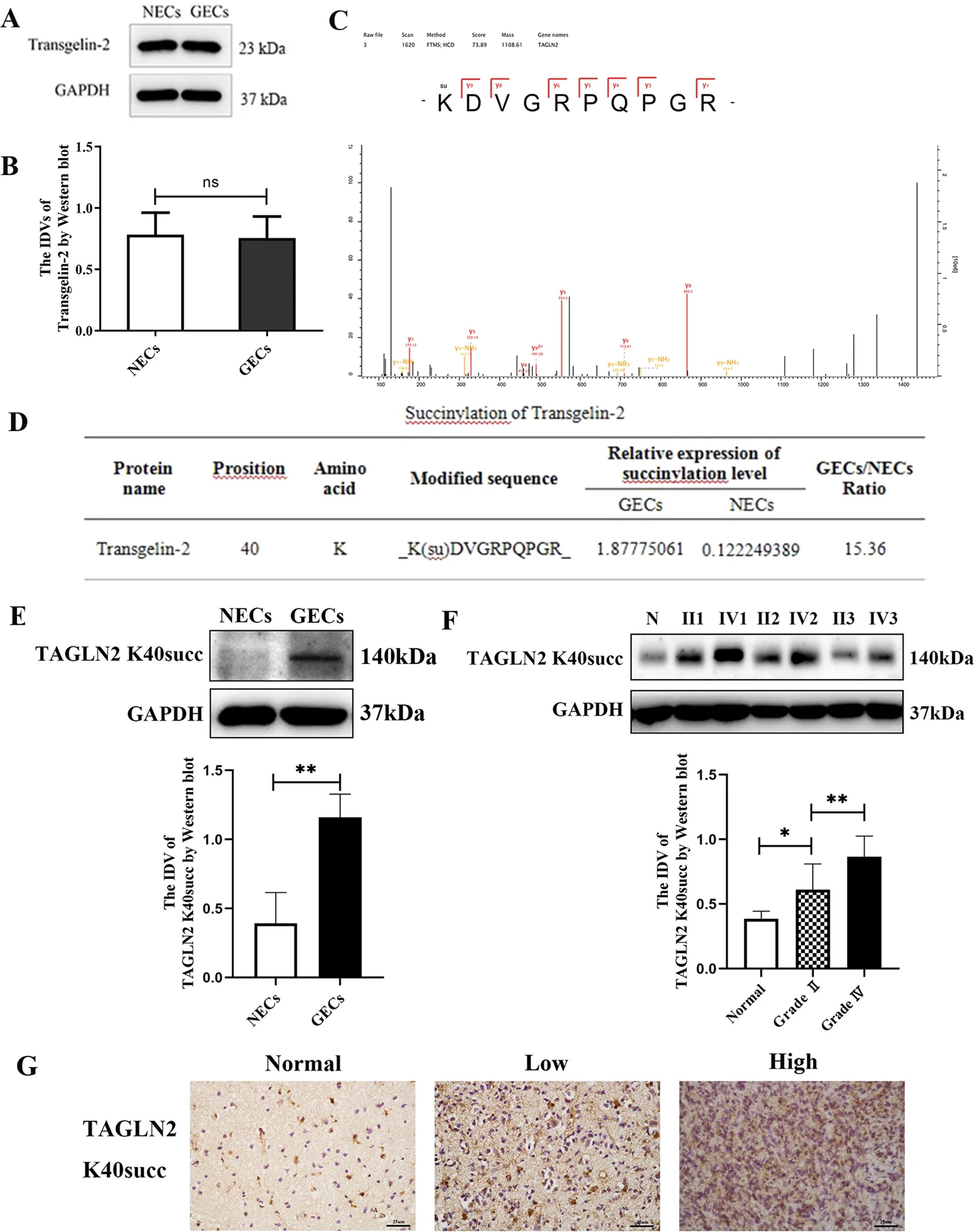
Zhang, X. et al. Cancer Gene Ther. 2022.
MtoZ Biolabs, leveraging the Orbitrap Fusion Lumos mass spectrometry platform and optimized proteomics analysis strategies, provides global researchers with high-quality Succinylation Proteomics Analysis Service. We help researchers explore the biological functions of succinylation modifications, advancing disease research and precision medicine development. For more information or to submit samples, feel free to contact us anytime.
MtoZ Biolabs, an integrated chromatography and mass spectrometry (MS) services provider.
Related Services
Histone Succinylation Analysis
How to order?







