Resources
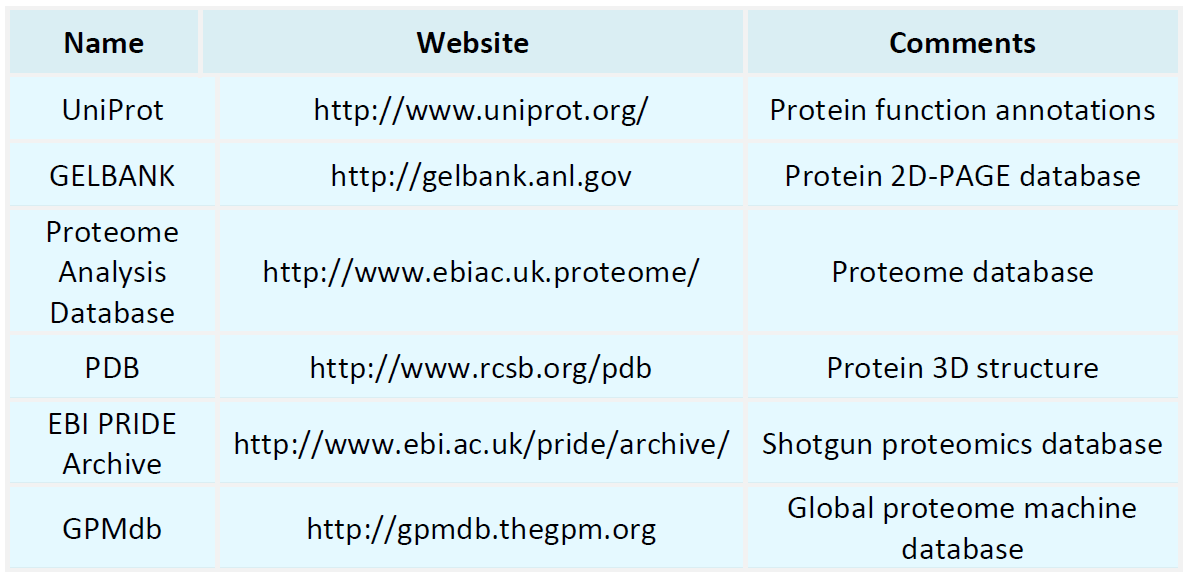
Proteomics Databases
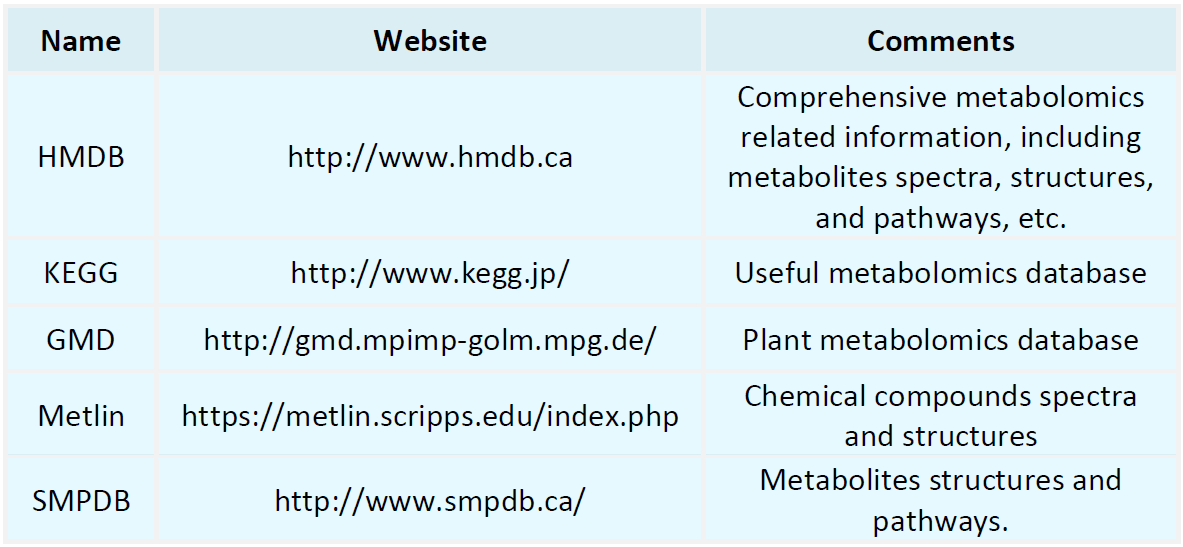
Metabolomics Databases
-
• Mass Spectrometry-Based Metabolomics
Mass spectrometry-based metabolomics is a scientific methodology that employs mass spectrometry for the qualitative and quantitative analysis of metabolites in biological samples. As a key tool in life sciences, metabolomics provides dynamic insights into biochemical reactions by profiling changes in small-molecule metabolites. This approach enables scientists to elucidate metabolic pathways, biochemical networks, and physiological states, offering valuable perspectives on organismal function. Beyon......
-
LC-MS/MS metabolomics integrates liquid chromatography with tandem mass spectrometry to enable comprehensive identification and quantification of small-molecule metabolites in biological samples. Liquid chromatography facilitates the efficient separation of complex biological matrices, allowing metabolites of varying chemical properties and concentrations to be introduced into the mass spectrometry system in a controlled manner, thereby enhancing resolution and sensitivity. Mass spectrometry then dete......
-
• Liver Proteomics: Insights into Function and Disease
Liver proteomics is a scientific discipline that employs proteomics technologies to systematically investigate the composition, structure, function, and regulatory mechanisms of proteins in liver tissues. As a crucial metabolic organ in both humans and animals, the liver plays a central role in biochemical processes such as substance metabolism, detoxification, immune regulation, and plasma protein synthesis. Liver proteomics research enables the elucidation of the molecular mechanisms underlying thes......
-
Glycosylation analysis is a specialized technique for investigating protein glycosylation, a post-translational modification in which sugar moieties are enzymatically attached to proteins. This modification is widespread and structurally diverse in biological systems, influencing protein conformation, stability, function, and molecular interactions. Glycosylation analysis is critical due to its extensive biological roles and clinical significance, including protein function regulation, cell-cell recog......
-
IPA analysis proteomics is an advanced bioinformatics tool designed to interpret complex proteomics data. By mapping mass spectrometry-identified proteins onto established biological networks, signaling pathways, and disease mechanisms, IPA analysis proteomics facilitates the elucidation of protein interactions and their functional roles in biological systems. This approach has broad applications in biomedical research, including studies on cancer, immune disorders, neurodegenerative diseases, and met......
-
Fish proteomics is a scientific discipline that employs high-throughput proteomics technologies to systematically investigate the composition, structure, post-translational modifications, and interactions of proteins in fish. As a crucial branch of proteomics in aquatic biology, fish proteomics facilitates the analysis of fish physiological and biochemical processes while elucidating their molecular responses to environmental changes, nutritional regulation, immune challenges, and evolutionary pressur......
-
• Identify Protein from Amino Acid Sequence
The ability to identify protein from amino acid sequence is a fundamental technique in proteomics research. By analyzing the composition and sequential arrangement of amino acids, this approach provides critical insights into protein structure and function. It plays a pivotal role in protein identification, functional characterization, post-translational modification (PTM) analysis, and the discovery of novel biomarkers. Given that protein function is inherently dictated by its amino acid sequence, th......
-
• Hypothetical Protein Analysis
Hypothetical protein analysis is a key research strategy in proteomics, designed to identify and characterize proteins predicted to exist but not yet confirmed by experimental evidence. These proteins are typically inferred from genome annotations, transcriptomic data, or computational models predicting their potential presence under specific biological conditions. By integrating advanced mass spectrometry techniques with computational bioinformatics, researchers can systematically detect and characte......
-
• Identification of New Drug Targets
The identification of new drug targets is a critical phase in drug discovery, aiming to identify and validate molecular targets that interact with therapeutic compounds and regulate specific physiological or pathological processes. These targets, including proteins, RNA molecules, receptors, and enzymes, play fundamental roles in disease onset and progression. Through systematic target identification, scientists can pinpoint key molecules associated with specific diseases and further investigate their......
-
• Global Analysis of Protein Structural Changes in Complex Proteomes
The global analysis of protein structural changes in complex proteomes is a critical yet challenging task for understanding biological processes and disease mechanisms. This approach provides insights into protein function under various physiological and pathological conditions and elucidates their roles in cellular pathways. Investigating protein structural changes enables the identification of disease mechanisms, discovery of potential therapeutic targets, and optimization of drug design. Furthermor......
How to order?







