Resources
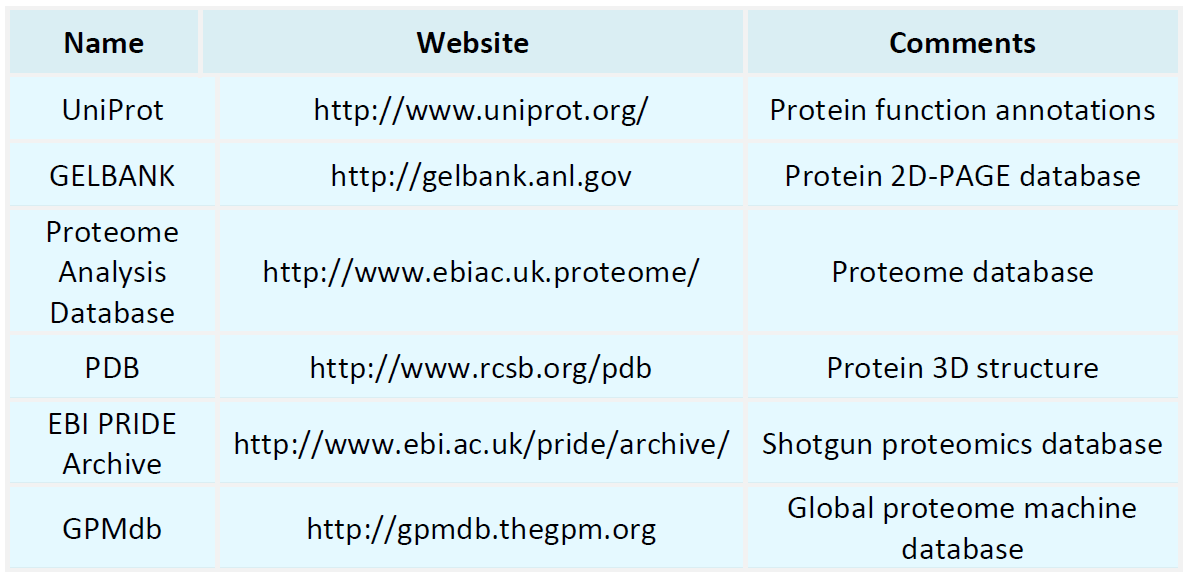
Proteomics Databases
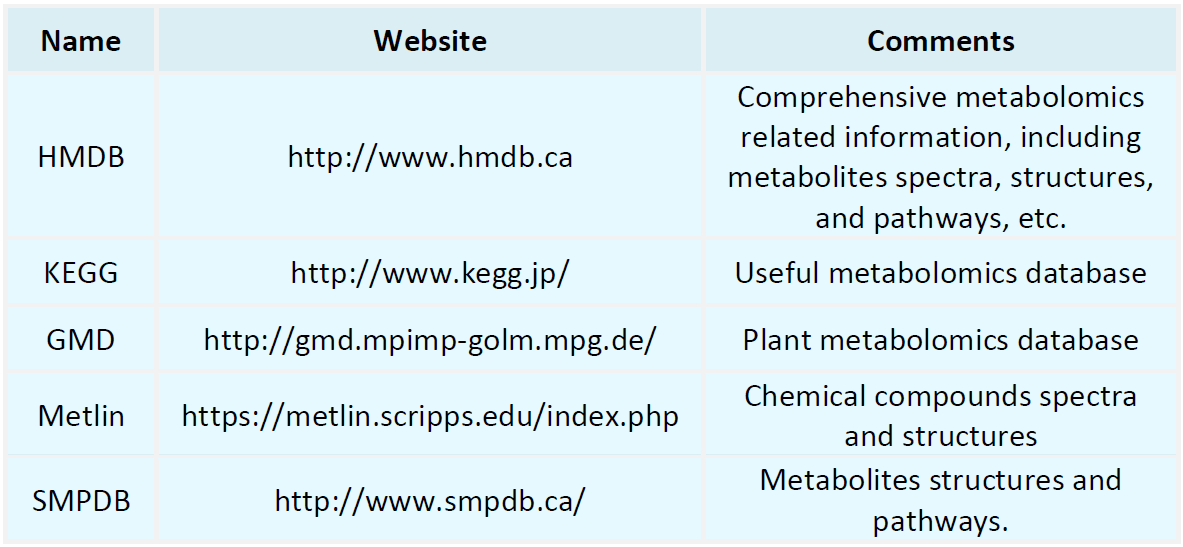
Metabolomics Databases
-
• How to Interpret Circular Dichroism Analysis Results?
Circular dichroism (CD) spectroscopy is a commonly used technique in biopharmaceutical analysis, which can be used to study the structure and conformational changes of biomolecules. By measuring the absorption of samples to left-handed and right-handed circularly polarized light at different wavelengths, we can obtain a CD spectrum.
-
• Advances in Circular Dichroism Analysis: Unveiling Structural Information from Spectra
Circular dichroism spectroscopy is an important biophysical analysis technique that reveals the structure and conformational information of molecules by measuring their optical activity at different wavelengths. In recent years, with the continuous development of technology, circular dichroism analysis has made many new advances in the field of biopharmaceuticals.
-
• Agricultural Large Sample Proteomics Solution
MtoZ Biolabs offers a comprehensive solution for agricultural large sample proteomics analysis, utilizing cutting-edge Orbitrap Astral mass spectrometry technology. This approach involves several key stages: Automated Sample Processing: We ensure that the biological samples from various conditions (e.g., different treatments or control groups) are efficiently processed with automated systems. This step includes protein extraction, quantification, and normalization, ensuring high throughput and consistency.
-
• MtoZ Biolabs: Deep Blood 4D-DIA Proteomics Service
MtoZ Biolabs introduces Deep Blood 4D-DIA (Data-Independent Acquisition) Proteomics, an advanced technology built to overcome the inherent challenges in blood proteomics research. By integrating the power of 4D-DIA and nanomagnetic bead-based enrichment techniques, we provide deep and precise analysis of blood samples, especially targeting low-abundance proteins. This approach enables comprehensive proteomic profiling, unlocking new insights into disease pathways and biomarker discovery.
-
• How to Optimize Mass Spectrometry for KEGG Enrichment Analysis in Proteomics
Introduction Proteomics is a scientific field that studies the composition, structure, function, and interactions of all proteins in organisms. The development of proteomics has provided important tools and methods for a deeper understanding of biological processes and disease mechanisms in organisms. Among them, mass spectrometry analysis, as one of the core technologies in proteomics research, plays a crucial role.
-
• How to Desalt Proteins in Mass Spectrometry?
Protein mass spectrometry is a commonly used biophysical analysis technique that helps scientists study the structure and function of proteins. However, before conducting protein mass spectrometry analysis, it is usually necessary to remove salts from the sample to avoid interference with the mass spectrometry signals.
-
• Exploring HCP Diversity: Unraveling Virus-Host Interaction Mechanisms
Host cell protein (HCP) residuals are common impurities in the manufacturing process of biopharmaceuticals. They are proteins from the host cells used in the bioreactor system, co-expressed and present in the final drug formulation. The presence of HCP residuals can potentially impact the quality and safety of the drug, making it important to explore and analyze the diversity of HCP residuals.
-
• Host Protein Residual Validation Guide: Effective Sample Analysis
Host protein residual verification is an important step in the development of biological drugs, used to confirm the presence of host cell-derived host protein residuals in the drug formulation. Host proteins refer to the proteins produced by host cells (usually mammalian cells) during the production of biological drugs. These host proteins may remain in the final drug formulation, potentially affecting the quality and safety of the drug.
-
• Detection of Membrane Proteins Based on Differential Scanning Fluorimetry
Membrane proteins play crucial roles in cellular processes, including signal transduction, transport, and cell communication. Their detection and analysis are vital for understanding their functions and implications in health and disease. Differential Scanning Fluorimetry (DSF) has emerged as a powerful technique for studying membrane proteins, offering insights into their stability and interactions.
-
• Dynamic Proteomics Analysis Using Shotgun Proteomics Techniques
Dynamic proteomics focuses on understanding the temporal changes in protein expression, modification, and interaction within cells, in response to various stimuli or under different physiological conditions. This field aims to map out the dynamic processes that govern cellular functions and adaptations. Shotgun proteomics, with its ability to identify and quantify thousands of proteins simultaneously, is particularly well-suited for dynamic proteomics studies.
How to order?







