Protein Active Component Identification and Quantification
Protein-based macromolecular drugs, typically organic compounds with molecular weights exceeding 1000, depend on cellular biosynthesis and are also known as biologics. Compared to traditional small molecule drugs, protein drugs are characterized by their high potency, strong specificity, low toxicity, clear biological function, and conducive to clinical application. Protein drugs are the fastest-growing class of drug molecules with more than 250 protein drugs clinically used in a variety of indications. Advances in recombinant technology have not only paved the way for an unlimited supply of proteins, but have also improved protein drugs' safety by eliminating viral transmission that can occur with proteins isolated from natural sources. Identifying the key proteins that influence disease progression can in turn develop their therapeutic potential, and understanding the disease at the molecular level can aid in the development of protein-based therapies.
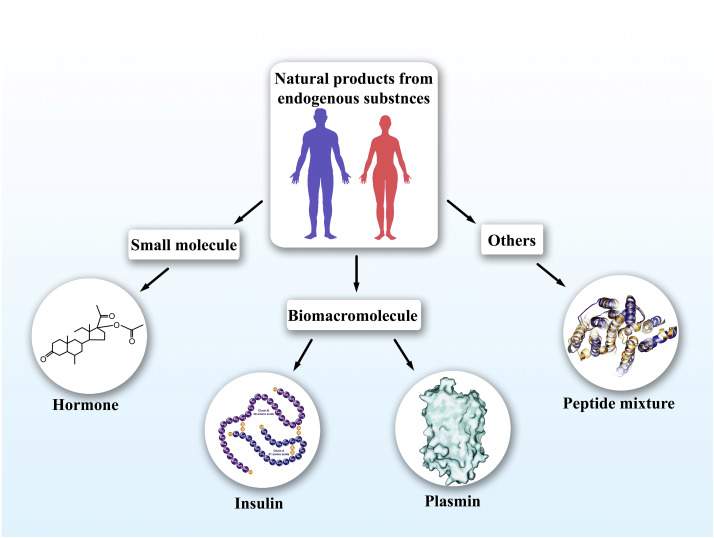
Figure 1. Pharmaceutical Strategies for the Discovery of Natural Products from Endogenous Substances [1]
Protein drugs include recombinant proteins (enzymes, growth factors, cytokines, etc.), genetic engineering drugs, monoclonal antibodies and genetic engineering antibodies, recombinant vaccines, etc. Recombinant protein drugs refer to the use of recombinant DNA technology to modify the target protein, and use a specific vector to insert the target gene into an appropriate host cell to express the target protein. Then biologically active protein products can be prepared through extraction and purification technology, which is used to make up for the lack of corresponding functional proteins in the body caused by congenital gene defects or acquired diseases, such as recombinant acid α-glucosidase (rhGAA) and factor VIII (FVIII) for Pompe disease and hemophilia A, as well as cytokine-based therapies such as interferon β for multiple sclerosis. In 1982, the first recombinant protein drug, recombinant human insulin, was launched. In 1985 and 1986, the first genetically recombinant human growth hormone and recombinant human interferon were launched in the world. At present, after years of development, recombinant protein drugs have become the most important class of products in the field of modern biopharmaceuticals, accounting for more than one-third of the market share of biological drugs.
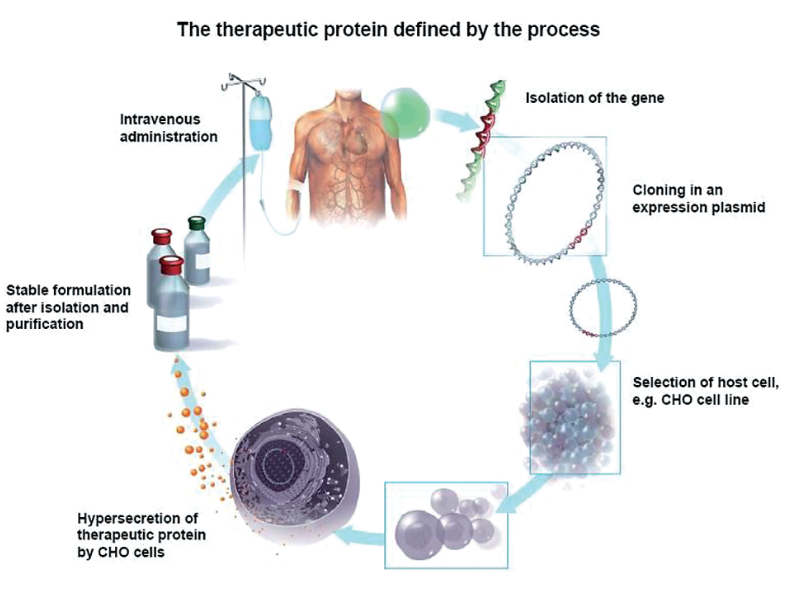
Figure 2. Application of Recombinant Therapeutic Proteins in the Treatment of Diseases [2]
In addition, monoclonal antibody-based drugs have been developed. Most approved antibodies are in IgG form, but several alternatives are emerging, including antibody-drug conjugates (ADCs) and other antibody fragments, including domain antibodies such as nanobodies, as well as bispecific antibodies (BsAbs), IgG cocktails, and antibody fusion proteins. Antibodies are most commonly used to treat cancer, autoimmune, and chronic inflammatory diseases, such as adalimumab (Humira), Fc-Fusion protein, etanercept (Enbrel) and Eloctate (Fc-FVIII) for rheumatoid arthritis, and immune checkpoint inhibitors such as pembrolizumab (Keytruda) and atezolizumab (Tecentriq), among several other antibody products. At the same time, antibody therapies are also being extended to a wider field, including infectious diseases, blood diseases, neurological diseases, eye diseases, and metabolic diseases, musculoskeletal diseases.
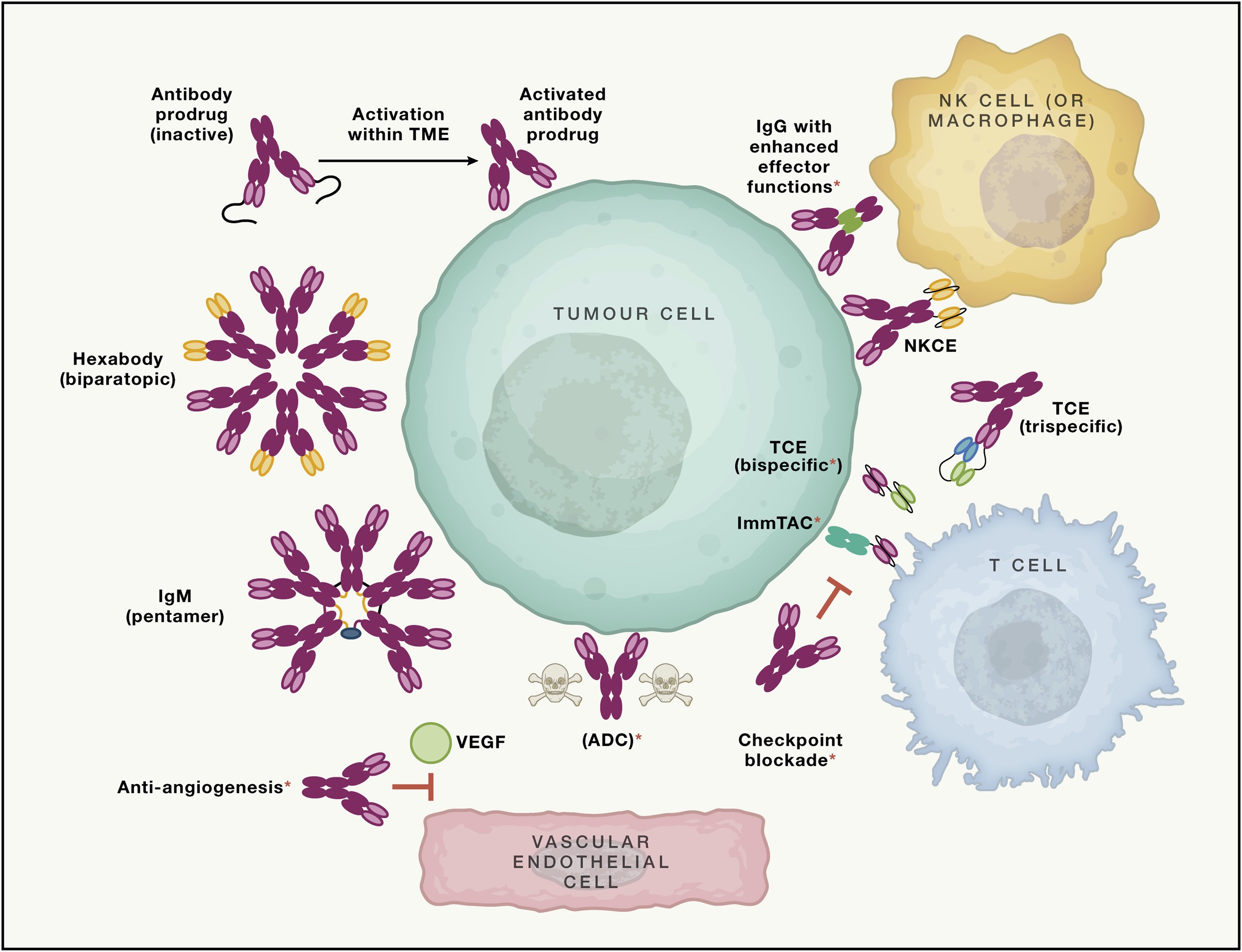
Figure 3. Strengthens Antibodies for Cancer Treatment [3]
Complex biological macromolecules that exceed the molecular weight of "traditional" small molecule drugs by thousands of times. Biopharmaceuticals require highly complex analytical workflows for their analysis and characterization. Most large molecule drugs are prone to sequence changes and inconsistent biotransformation during the production process. These changes have implications for the efficacy, bioavailability, and safety of the drug. In protein research, it refers to the characteristics of proteins, including structure, physicochemical and activity. Accurate amino acid sequences, molecular weights, valence changes, glycosylation, aggregation levels, and oxidation levels are all critical elements for protein drug characterization. Chromatography and mass spectrometry play an important role in the characterization of macromolecular proteins.
In general, the physicochemical parameters of protein macromolecules include the determination of primary structure, composition, and physicochemical properties. Information about the primary structure can be determined in combination with a range of results such as peptide maps, C-terminal and N-terminal sequences, amino acid composition, and sequences. The higher-order structure of proteins is also important because they have a significant impact on the activity of the drug product. Data on higher-order structures can be obtained by spectroscopic methods such as circular dichroism (CD) and nuclear magnetic resonance (NMR). Other physicochemical property data such as molecular weight or size, isomerization type, extinction coefficient, and electrophoretic spectra should also be acquired. Data for identification, homogeneity, and purity can also be collected using a variety of techniques such as liquid chromatography (LC), mass spectrometry (MS), or liquid chromatography-MS (LC-MS).
The purity and peptide profile of protein polypeptides were analyzed by high performance liquid chromatography (HPLC), so as to determine the protein purity and peptide distribution after enzymatic hydrolysis. Purity can be used to analyze impurities and content in industrial protein and peptide samples, and peptide mapping can be used to identify peptide distributions and observe consistency from batch to batch.
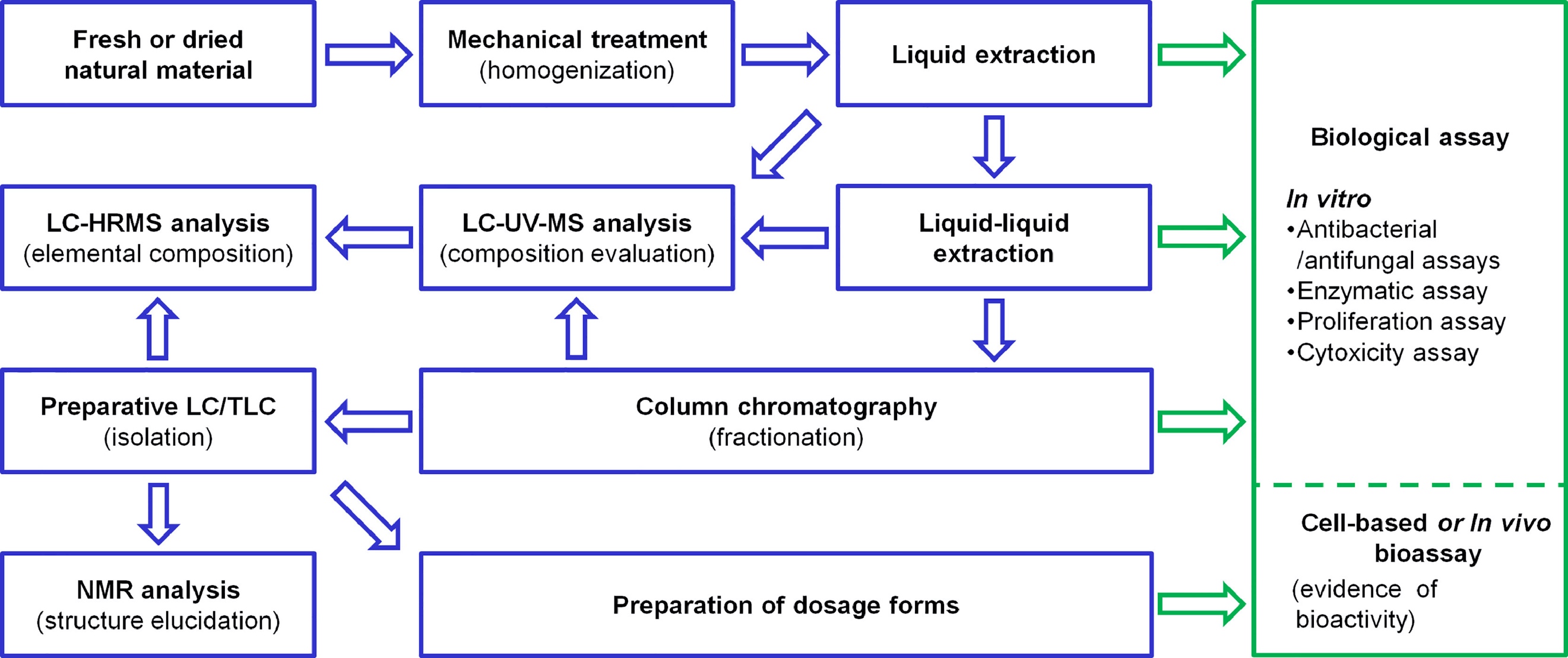
Figure 4. A Comprehensive Approach to Traditional Procedures for Isolating, Purifying, and Characterizing Biological Activity [4]
Peptide profiling of protein and peptide drug molecules is a key element of drug quality control, which is of great significance for the theoretical sequence confirmation, high-level structure analysis, and biological function study of protein and peptide drugs. Peptide spectroscopy can confirm the theoretical sequence and modification of protein polypeptides, and the common modifications of simple samples are deamidation and oxidation, and the common modifications of antibodies are deamidation, oxidation, N-terminal cyclization, C-terminal K deletion, glycation, etc., and peptide spectra can identify the localization of disulfide bonds. MS-based proteomics enables the assessment of protein properties and their functional regulation at multiple levels of proteins in biological specimens. The evolution of various aspects of the workflow (speed, sensitivity, precision, and robustness) has allowed MS-based proteomics to reach a level that allows for the streamlining of nearly complete proteomic analysis in a matter of hours, including dissecting customized methods for protein abundance, turnover, subcellular localization, interactions, and modifications for target discovery and validation.
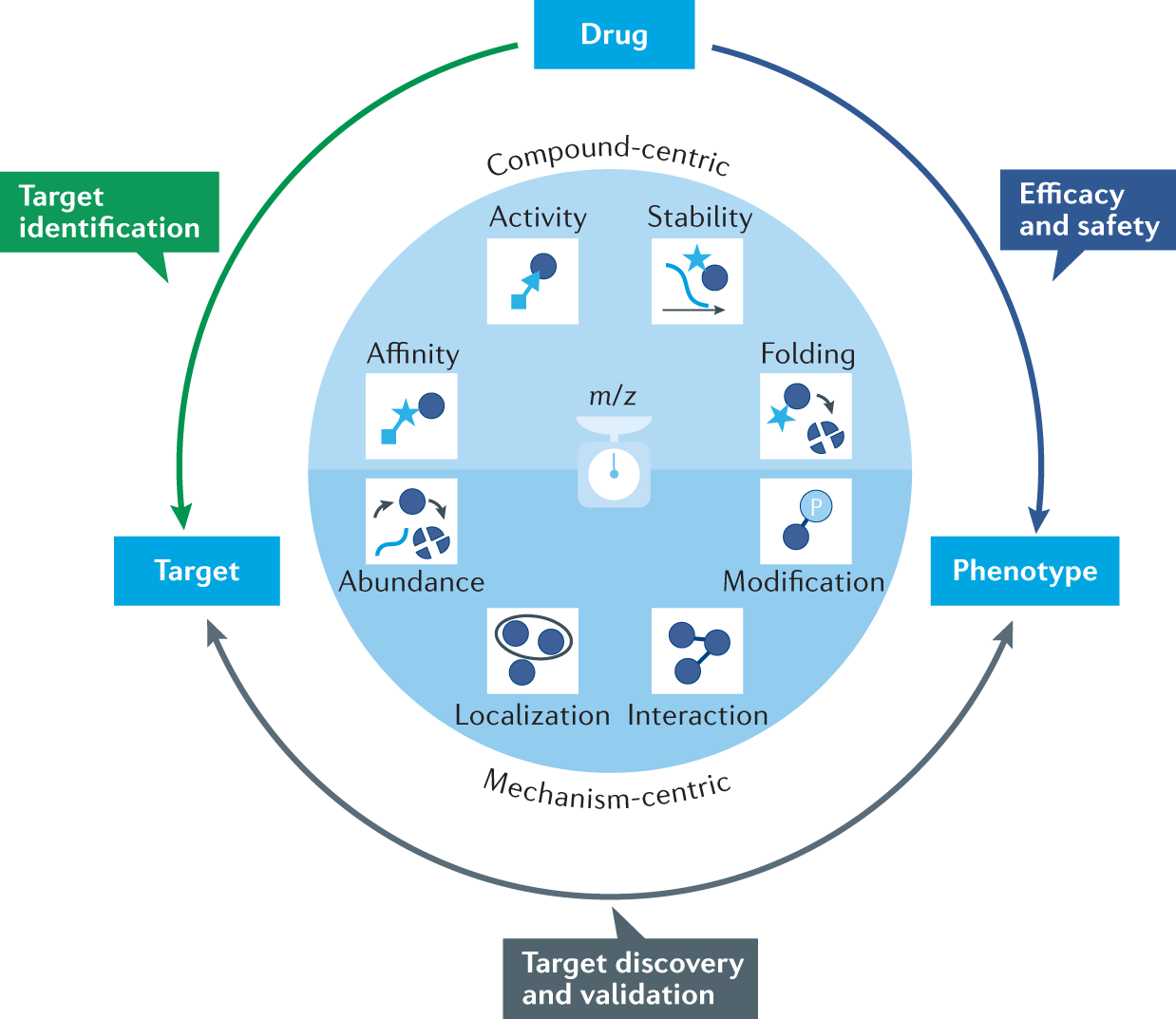
Figure 5. Proteomics Methods for Target Identification, Target Validation, and Monitoring Safety and Efficacy [5]
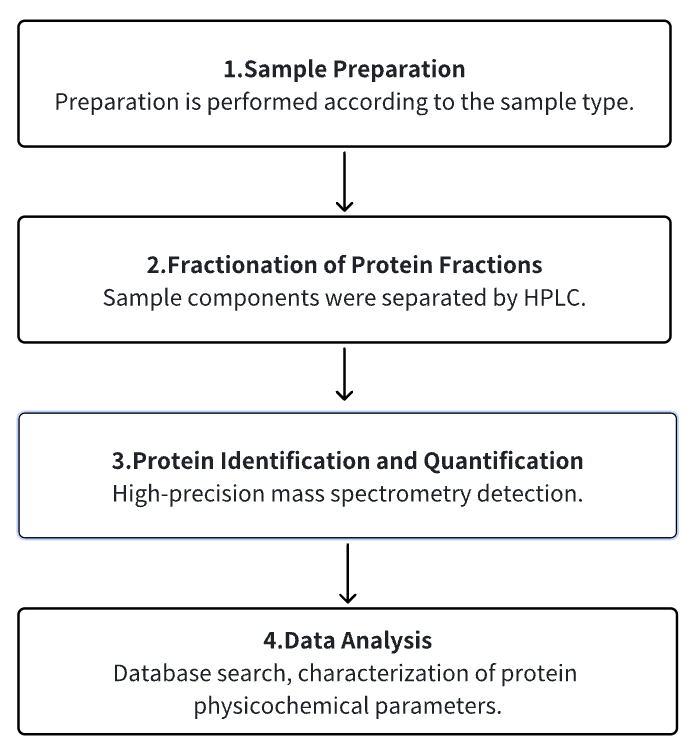
Analysis Workflow
1. Sample Preparation
2. Fractionation of Protein Fractions
3. Protein Identification and Quantification
4. Data Analysis
Service Advantages
1. Identification/ Quantification/ Modification Identification of Multi-Type Sample-Derived Proteins, etc.
2. High-Confidence, High-Precision MS Detection
3. Comprehensive Bioinformatics Analysis
Sample of Results
1. SP3 host cell protein (HCP) monitoring in AAV-based gene therapy products using LC-MS/MS. [6]
Residual HCPs are a key quality attribute for biotherapeutics products. Workflows have been developed for reliable HCP detection in monoclonal antibodies and recombinant proteins, which helps optimize the process to improve product stability and safety, and allows acceptance limits to be set for HCP content. However, the detection of HCP in gene therapy products such as adeno-associated virus (AAV) vectors have been limited. Therefore, there are studies using SP3 sample preparation followed by LC-MS analysis for HCP analysis in various AAV samples.
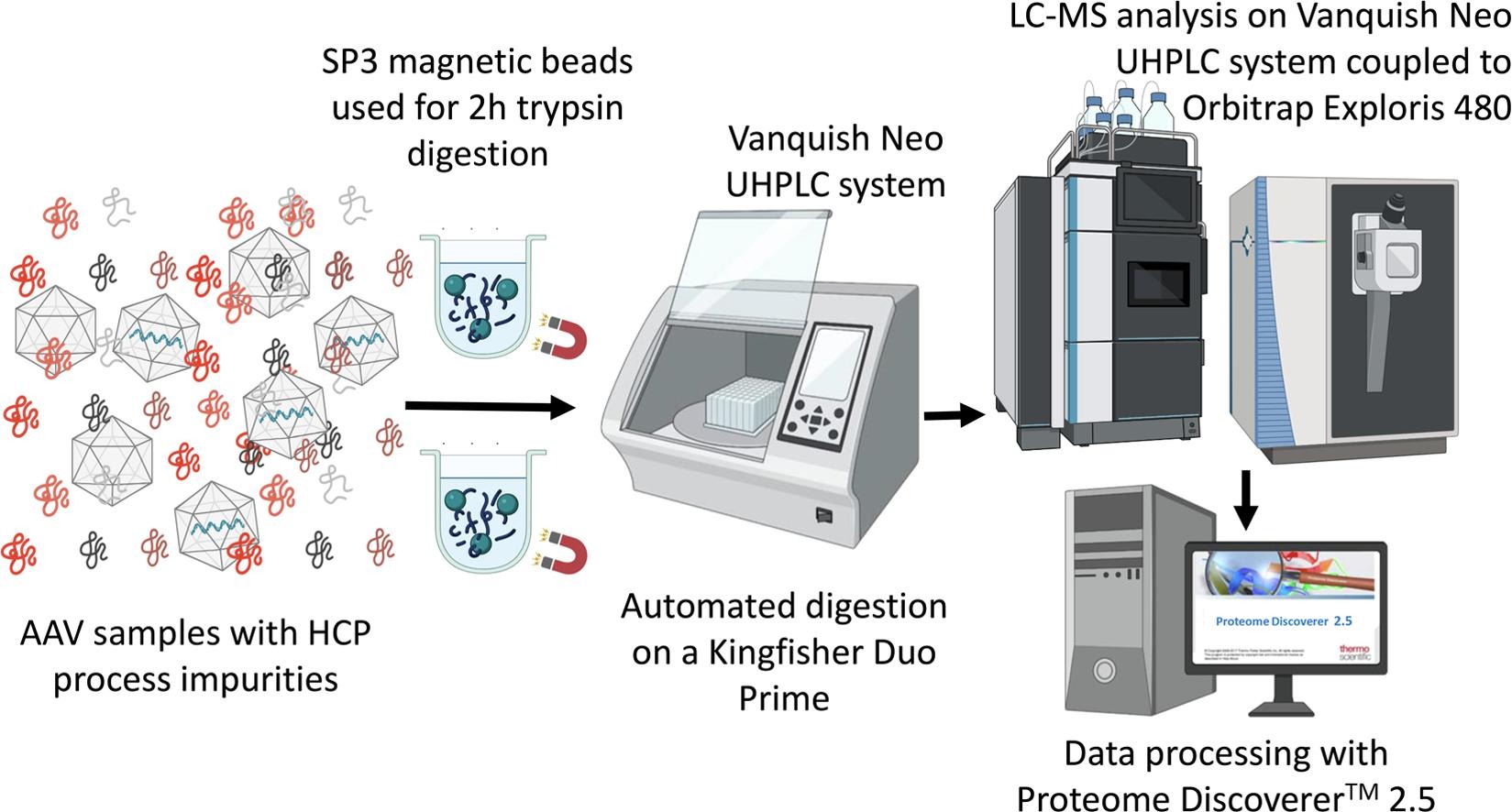
Figure 6. LC-MS/MS Was Used to Detect Protein Residues in AAV Gene Therapy Products
2. Oxidative and reduction analysis of therapeutic recombinant human interleukin-15 by HPLC and LC-MS. [7]
Recombinant human interleukin-15 (rhIL-15) has been extensively studied as an important immunostimulatory agent for T lymphocytes and NK cells in tumor immunotherapy or as a vaccine adjuvant. However, due to the lack of effective and precise analytical methods to characterize trace by-products (typically redox and deamidation), the production level of rhIL-15 lags far behind its growing clinical needs. In order to improve the production and quality control of rhIL-15, an panded resolution reversed-phase HPLC (ExRP-HPLC) method was developed to quickly and accurately analyze the oxidation and reduction by-products that may occur during the purification of rhIL-15. First, we developed the RP-HPLC method to separate rhIL-15 fractions at different oxidation or reduction levels, and then determined the redox state of each peak by measuring intact mass with a high-resolution mass spectrometer (Ultra performance liquid chromatography-MS, UPLC-MS).
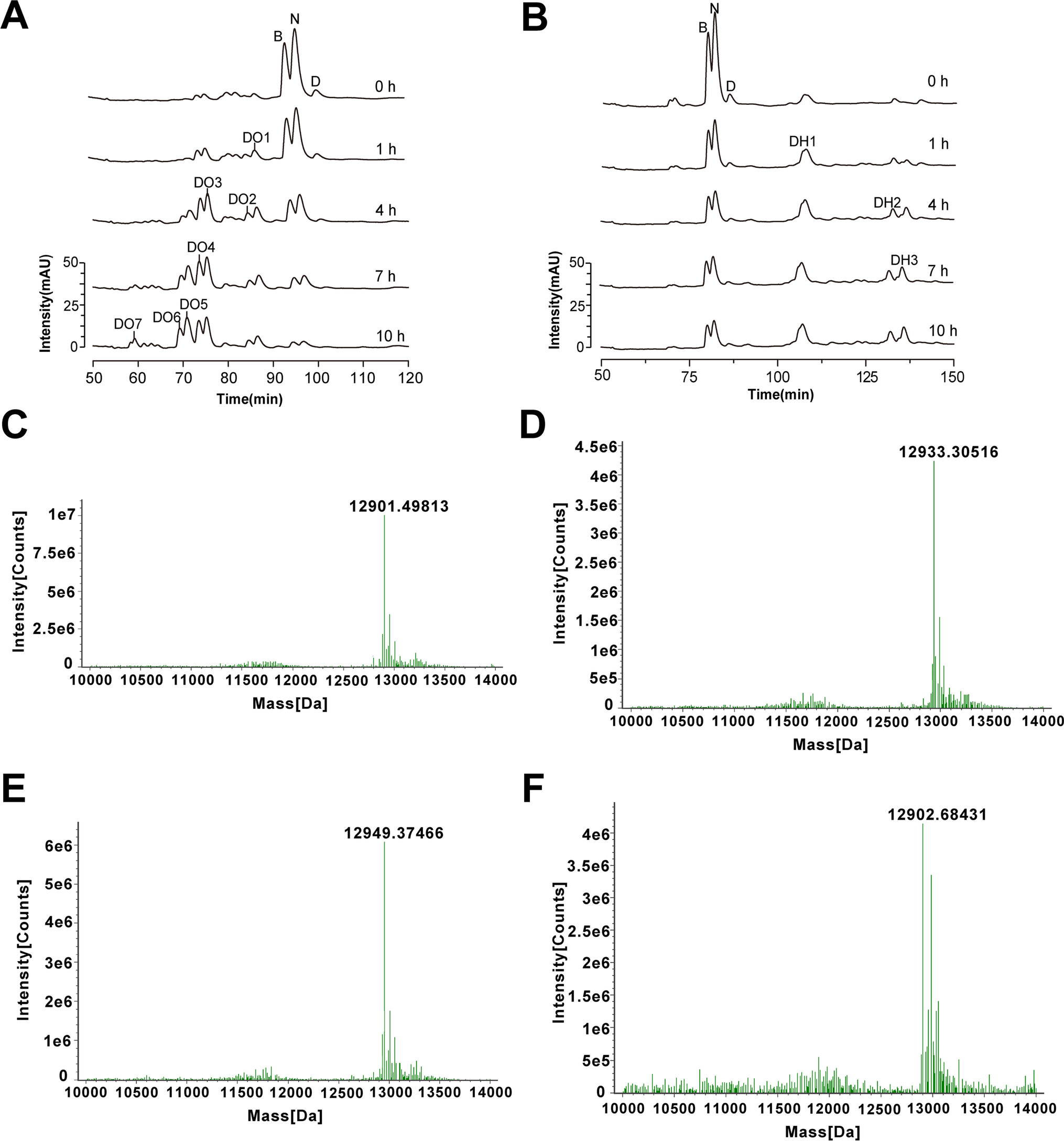
Figure 7. The Oxidation/Reduction Products of Deamidated RhIL-15 Were Analyzed Using ExRP-HPLC and UPLC-MS
3. Liquid chromatography-tandem mass spectrometry (LC-MS/MS) was used to determine natalizumab in serum and cerebrospinal fluid of patients with multiple sclerosis. [8]
Natalizumab is a humanized recombinant monoclonal IgG4 antibody used for the treatment of multiple sclerosis. The common methods for quantification of natalizumab and anti-natalizumab antibodies are enzyme-linked immunosorbent assay (ELISA) and radioimmunoassay, respectively. Due to their similarity to human plasma immunoglobulins, the measurement of therapeutic monoclonal antibodies can be challenging. Recent developments in MS enable the analysis of a wide variety of large protein molecules. The purpose of this study is to develop an LC-MS/MS method for the determination of natalizumab in human serum and cerebrospinal fluid and its clinical application. For successful quantification, it is necessary to find the specific peptide sequence in natalizumab. The immunoglobulin was treated with dithiothreitol and iodoacetamide, cleaved into a short specific peptide with trypsin, and assayed on a UPLC-MS/MS system, testing for accuracy and precision within and between assays at four concentration levels. The accuracy is determined by the coefficient of variation and is in the range of 0.8-10.2% and the accuracy is in the range of 89.8-106.4%. Concentrations of natalizumab in patient samples ranged from 1.8 to 193.3 μg/mL. The method was validated according to the European Medicines Agency (EMA) guidelines and met all acceptance criteria for accuracy and precision, making it suitable for clinical applications.
Figure 8. Comparison of Serum-Free Natalizumab with Serum Natalizumab
Sample Submission Requirements
1. Whenever possible, provide processed and purified samples.
2. Try to avoid contamination by impurities.
Services at MtoZ Biolabs
1. Complete experimental procedures.
2. Relevant instrument parameters.
3. Raw MS data.
4. Detailed information report on the identification of protein macromolecules (including accurate molecular weight determination, primary sequence confirmation, sequence coverage/peptide profiling, modification profiling, disulfide bond analysis, etc.).
Applications
1. Recombinant Protein Drugs
At present, recombinant protein drugs mainly include recombinant insulin, recombinant interferon, recombinant coagulation factor, recombinant erythropoietin, recombinant granulocyte colony-stimulating factor, enzyme replacement recombinant protein drugs, recombinant growth hormone, etc. Among them, recombinant insulin occupies an important position in recombinant protein drug market, and recombinant interferon, recombinant erythropoietin and other sub-products have great development potential, especially long-acting drugs. At present, the most popular recombinant proteins are mainly cytokines, and the cytokines mainly include the following:
(1) Interleukin (IL): a class of cytokines produced by and acting on a variety of cells. Because it was originally produced by white blood cells and functions among white blood cells, it got its name, and it is still used today.
(2) Interferon (IFN): It has the ability to interfere with viral replication, hence the name. It has a very wide range of biological activities, plays an important role in immune response and immune regulation, and is also one of the main pro-inflammatory cytokines.
(3) Tumor necrosis factor (TNF): Named because it can directly kill tumor cells in vitro and in vivo. There are about 30 members of the family. It mainly includes TNF-α and TNF-β.
(4) Colony stimulating factor (CSF): a group of cytokines that can selectively stimulate the proliferation and differentiation of hematopoietic progenitor cells in vitro and in vivo to form a colony of a certain lineage, including macrophage CSF, granulocyte CSF, macrophage/granulocyte CSF, stem cell factors, etc.
(5) Growth factor (GF): A class of cytokines that can mediate the growth and differentiation of different types of cells, according to their functions and different cells, they are named transforming growth factor (TGF), nerve growth factor (NGF), epidermal growth factor (EGF), vascular endothelial cell growth factor (VEGF), fibroblast growth factor (FGF), etc.
(6) Chemokines: A family of cytokines that have chemotaxis effects on different target cells, which can be secreted by leukocytes and certain tissue cells, and is a protein family with more than 60 members. Most of the members contain 4 conserved cysteines (C), which can be divided into four subfamilies according to the arrangement of N-terminal cysteine: CXC, CC, C, and CX3C.
2. ADCs
ADCs have become an important class of drugs in clinical research for cancer treatment. ADCs are a class that utilizes the targeted selectivity of monoclonal antibodies (mAbs) to achieve targeted delivery of cytotoxicity. As a result of this targeting, ADCs selectively eliminate tumor cells that overexpress the target antigen while limiting the toxicity of the drug to normal healthy tissues. For example, brentuximab (SGN-35) is used to treat CD30-positive malignancies (Hodgkin lymphoma), and trastuzumab emtansine (T-DM1) is used in human epidermal growth factor receptor 2 (HER2)-positive metastatic breast cancer. The clinical efficacy of an ADC depends on the specificity of the target, the adapter and drug class, in vitro and in vivo stability, the potency, and the distribution of the drug class and the number of small molecule conjugates. Therefore, it is particularly important to understand the physicochemical properties of ADCs and select appropriate analytical techniques for their evaluation and quality monitoring during manufacturing and storage.
FAQ
Q1: In addition to physicochemical parameters, what kind of analysis can be performed on protein samples?
The determination of biological activity is important because it determines the therapeutic effect of the drug. The data on biological activity give functional information, and indirectly also give protein folding information. Methods used to determine biological activity include animal-based bioassays, cell culture assays, and biochemical assays. The results of the bioassay should be expressed as viability units and calibrated with a commercial or homemade reference material as a control. If the product is an antibody, its immunochemical properties should be characterized in its entirety. Avidity, viability, and immune activity of antibodies should be determined by antibody binding assays to pure antigens and specific types of antigens. The immunochemical properties of proteins can be used to determine their identity, homogeneity, or purity.
References
[1] Jaegle M, Wong EL, Tauber C, Nawrotzky E, Arkona C, Rademann J. Protein-Templated Fragment Ligations-From Molecular Recognition to Drug Discovery. Angew Chem Int Ed Engl. 2017 Jun 19;56(26):7358-7378. doi: 10.1002/anie.201610372. Epub 2017 May 31. PMID: 28117936; PMCID: PMC7159684.
[2] Dingermann T. Recombinant therapeutic proteins: production platforms and challenges. Biotechnol J. 2008 Jan;3(1):90-7. doi: 10.1002/biot.200700214. PMID: 18041103.
[3] Carter PJ, Rajpal A. Designing antibodies as therapeutics. Cell. 2022 Jul 21;185(15):2789-2805. doi: 10.1016/j.cell.2022.05.029. PMID: 35868279.
[4] Stavrianidi A. A classification of liquid chromatography mass spectrometry techniques for evaluation of chemical composition and quality control of traditional medicines. J Chromatogr A. 2020 Jan 4;1609:460501. doi: 10.1016/j.chroma.2019.460501. Epub 2019 Aug 30. PMID: 31515074.
[5] Meissner F, Geddes-McAlister J, Mann M, Bantscheff M. The emerging role of mass spectrometry-based proteomics in drug discovery. Nat Rev Drug Discov. 2022 Sep;21(9):637-654. doi: 10.1038/s41573-022-00409-3. Epub 2022 Mar 29. PMID: 35351998.
[6] Smith J, Strasser L, Guapo F, Milian SG, Snyder RO, Bones J. SP3-based host cell protein monitoring in AAV-based gene therapy products using LC-MS/MS. Eur J Pharm Biopharm. 2023 Aug;189:276-280. doi: 10.1016/j.ejpb.2023.06.019. Epub 2023 Jul 6. PMID: 37419424.
[7] Wang Y, Chen H, Zhao M, Feng L, Liu Z, Zeng Q, Shi W, Zhu W, Song L, Zhu J, Lu H. Oxidation and reduction analysis of therapeutic recombinant human interleukin-15 by HPLC and LC-MS. Appl Microbiol Biotechnol. 2023 May;107(10):3217-3227. doi: 10.1007/s00253-023-12508-1. Epub 2023 Apr 14. PMID: 37058229.
[8] Matlak P, Brozmanova H, Sistik P, Kacirova I, Hradilek P, Grundmann M. Liquid chromatography - tandem mass spectrometry method for determination of natalizumab in serum and cerebrospinal fluid of patients with multiple sclerosis. J Pharm Biomed Anal. 2023 Sep 20;234:115542. doi: 10.1016/j.jpba.2023.115542. Epub 2023 Jun 22. PMID: 37364452.
MtoZ Biolabs, an integrated chromatography and mass spectrometry (MS) services provider.
Related Services
Active Pharmaceutical Ingredients (APIs) Identification Service
How to order?







