PROTAC Molecules Activity and Efficacy Evaluate Service
PROTACs represent a novel strategy for protein degradation that has gained prominence recently. Utilizing bifunctional molecules, PROTACs enable the targeted ubiquitination and subsequent degradation of proteins via the UPS. PROTACs can not only offer potential therapeutic applications for a variety of conditions, including cancer, immune dysregulation, viral infections, and neurodegenerative diseases, but also provide a unique chemical knockdown tool for biological research in a catalytic, reversible, and rapid manner.
A PROTAC molecule comprises two functional parts: an E3 ligase recruiter and a ligand for the protein of interest (POI). By bringing the E3 ligase and POI into proximity, PROTACs initiate the process of ubiquitination, leading to the degradation of the target protein by the proteasome. This interaction enables the transfer of ubiquitin from the E3 ligase to the POI, marking it for recognition and breakdown into peptides by the proteasome. Both the E3 ligase and the target protein can bind simultaneously to a single PROTAC molecule. Through the orchestrated actions of E1, E2, and E3 ligases, ubiquitin is repeatedly transferred to the target, facilitating its polyubiquitination and subsequent degradation.
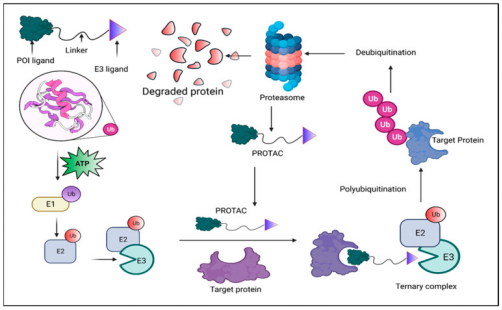
Figure 1. Principle of PROTAC-Mediated Degradation [4]
1. E3 Ligase Affinity Measurement
The binding specificity of E3 ligands is critical and should be optimized to enhance the formation of effective PROTAC complexes. It is recommended to employ binding assays such as fluorescence polarization or time-resolved fluorescence resonance energy transfer (TR-FRET) to confirm the presence of the E3 ligase binding motifs essential for PROTAC function.
2. Ternary Complex Formation Detection
Successful PROTAC function requires the strategic connection of the E3 ligase and the POI through a specifically designed linker, forming a ternary complex. One of the most important methods to assess ternary complex formation is proximity detection, such as TR-FRET or cross-linking mass spectrometry (XL-MS). TR-FRET uses long-lifetime fluorophores and integral time fluorescence to quantitatively analyze molecular binding and dissociation. This technique can be used to study the interactions between PROTACs and their POIs. XL-MS used for studying protein-protein interactions, can help visualize interaction regions by identifying protein complexes.
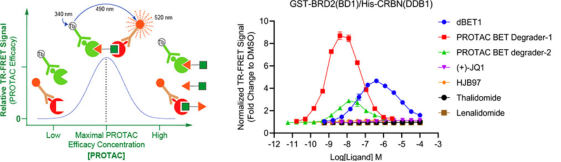
Figure 2. TR-FRET Measurement to Characterize PROTAC Ternary Complexes [5]
3. Target Protein Affinity Measurement
During PROTAC development and screening, assessing the binding affinity of the target protein ligand is essential. Platforms such as Pull-down assays, thermal shift assay, and thermal shift assay (TSA) are utilized for this purpose, providing valuable insights into the ligand-target interactions.
4. Protein Degradation Capability Testing
Protein degradation is the most important indicator of PROTAC compound activity. Various protein degradation analysis technology platforms, including Western Blot (WB), in cell western (ICW), meso scale discovery (MSD), homogeneous time resolved fluorescence (HTRF), enzyme linked immunosorbent assay (ELISA), HiBiT, etc., can test the target protein degradation capability of PROTAC molecules at different throughput levels. To determine whether the POI has been degraded, protein expression levels can be assessed by WB or immunoassay. Large-scale protein degradation analysis can be achieved using tandem mass spectrometry (MS/MS) and tandem mass tag (TMT) systems. On a smaller scale, single-cell analysis can be utilized. Additionally, TR-FRET detection can determine the lack of interaction between PROTAC and respective POIs to evaluate degradation.
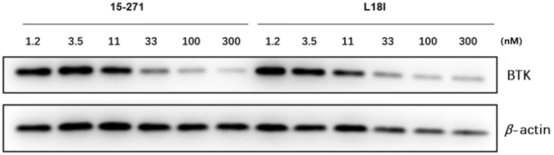
Figure 3. WB Detection of PROTACs' Efficiency in Degrading BTK [6]
Mass spectrometry-based proteomics is a discovery tool that allows researchers to understand the mechanisms of action of candidate drugs. When applied to PROTACs, these methods provide an unbiased perspective on binding, degradation selectivity, and mechanisms related to efficacy and safety. Specifically, global analysis experiments can identify direct degradation events and assess downstream pathway changes caused by degradation or off-target inhibition.
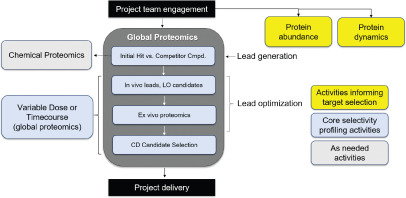
Figure 4. Applications of Proteomics in the PROTAC Field [7]
5. Ubiquitination Detection
Ubiquitination is a prerequisite for the recognition and degradation of target proteins by the proteasome, and it is a critical step in determining the success of PROTACs. Techniques such as WB, TR-FRET, ELISA, amplified luminescent proximity homogeneous assay-linked immunosorbent assay (AlphaLISA), immunoprecipitation (IP), etc., can be used to assess ubiquitination levels. TR-FRET detection allows for high-throughput screening to monitor PROTAC-induced ubiquitination in kinetic or endpoint readings. Multi-ubiquitinated proteins can be isolated from cell lysates using high-binding affinity resin and analyzed using ubiquitin enrichment kits. Proteomic analysis can also be an important tool for identifying proteins before and after modification.
6. In Vitro Solubility Test
The oral absorption of drugs occurs in the intestine, and thus the solubility in physiological solutions more closely resembles the real environment of PROTAC intestinal absorption. That is to say, poor solubility in phosphate buffer is not absolute, and attention should also be paid to the solubility of PROTACs in physiological solutions. Conventional solubility studies measure the solubility of compounds in pH 7.4 phosphate buffer, but for PROTAC molecules, measuring solubility in phosphate buffer alone is insufficient. Literature reports that the solubility of PROTAC molecules significantly increases in fasted state simulating intestinal fluid (FaSSIF) and fed state simulating intestinal fluid (FeSSIF), with the best solubility observed in FeSSIF.
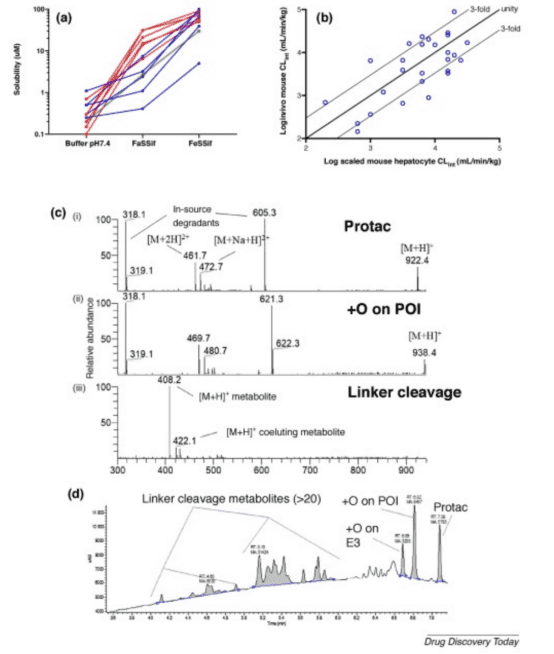
Figure 5. Biorelevant Solubility of PROTACs [8]
7. Cellular Assays
During the development and optimization of PROTAC drugs, it is essential to evaluate cellular responses such as proliferation, apoptosis, and the effects of drugs within living cells. Techniques including CellTiter-Glo, Caspase-Glo, LDH-Glo, and Wash-out assays are employed to further refine PROTAC formulations. Additionally, flow cytometry, microplate assays, and high-content screening are utilized to assess cellular activity, providing detailed insights into the physiological condition of cells following treatment with PROTAC molecules.
8. Cellular Permeability Assays
Permeability is another key parameter affecting drug absorption, with some PROTAC molecules needing to cross the cell membrane to bind to intracellular target proteins. Common in vitro permeability models include Caco2, MDCK, LLC-PK1, and PAMPA. PAMPA is a cell-free, simple phospholipid bilayer model used to measure passive permeability and has been shown to correlate with in vivo oral bioavailability. Given the simplicity of PAMPA, it allows for the assessment of passive membrane permeability without confounding factors such as active transport mechanisms present in cell-based models. Compared to cellular assays, one of the main advantages of this test is its relatively low cost and high throughput, which helps in making rapid decisions during the candidate analysis period for passive permeability. The use of cells such as Caco-2 or immortalized cell lines (typically of epithelial origin) for transcellular permeability testing has been widely implemented. These assays help predict oral absorption and select preclinical candidate drugs, although they are relatively lower in throughput and complexity compared to PAMPA. Regardless of the model, PROTAC molecules have shown low permeability, with higher molecular weight PROTACs more likely to be substrates for efflux transporters.
9. Drug Metabolism Testing
The in vitro metabolic stability test commonly uses liver microsomes metabolic stability (LMS) and hepatocyte metabolic stability (HMS). For PROTAC drugs, it is important to note that during the LMS testing process, based on their physicochemical properties and high protein binding, high protein binding may reduce their LMS clearance rate. For assessing the inherent metabolic stability properties, it is necessary to focus on the fraction of unconjugated (fu) and the minimum inhibitory concentration (mic) or replace it with the fraction unbound in plasma (fu, p) during the test, and the intrinsic clearance of unbound drug (Clint, u) is obtained by correcting for the intrinsic clearance (Clint) to purely look at the metabolic stability of the compounds and to provide support for subsequent in vivo-in vitro extrapolation (IVIVE). Additionally, HMS will be more stable compared to LMS due to the low permeability characteristics of the PROTAC molecule, which results in limited access to intramembranous metabolizing enzymes across the hepatocyte membrane, but this difference does not represent the strength of the correlation with in vivo clearance, and needs to be correlated with the results of the actual study. In the study of the metabolic stability and structural modification of PROTAC molecules, the molecule as a whole can be optimized and tested separately. Microsomes from different species (mouse, rabbit, dog, monkey, or human) can be used for compound liver stability testing.
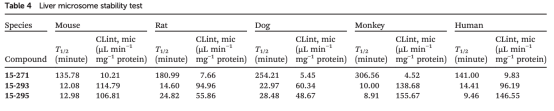
Figure 6. PROTAC Liver Stability Test Results [6]
Routine early distributive property tests mainly include in vivo intravenous (IV) and in vitro plasma protein binding (PPB), with in vivo IV testing being unremarkable. PPB is an important part of the drug screening process, based on the free drug hypothesis, assuming no transporter effects, the concentration of free drug in plasma equals the concentration of free drug in tissues. Thus, PPB testing can be used for PK/PD analysis based on plasma free drug concentrations, and for comparing the advantages of high or low free drug concentrations during the screening stage. PPB testing generally includes three methods: equilibrium dialysis, ultrafiltration, and ultracentrifugation. Among them, equilibrium dialysis is the most common testing method for conventional molecules, but PROTAC molecules are prone to issues such as adsorption by the dialysis membrane and recovery problems caused by solubility in this test system. Since both the equilibrium dialysis and ultrafiltration test systems contain dialysis membranes, they are not very suitable for PROTAC molecule PPB testing. In contrast, ultracentrifugation is more suitable for PROTAC analysis of PPB testing.
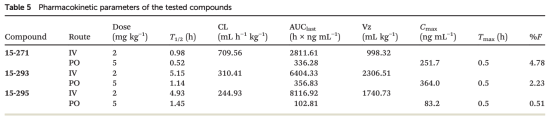
Figure 7. In Vivo Pharmacokinetics Testing of PROTACs in Mice [6]
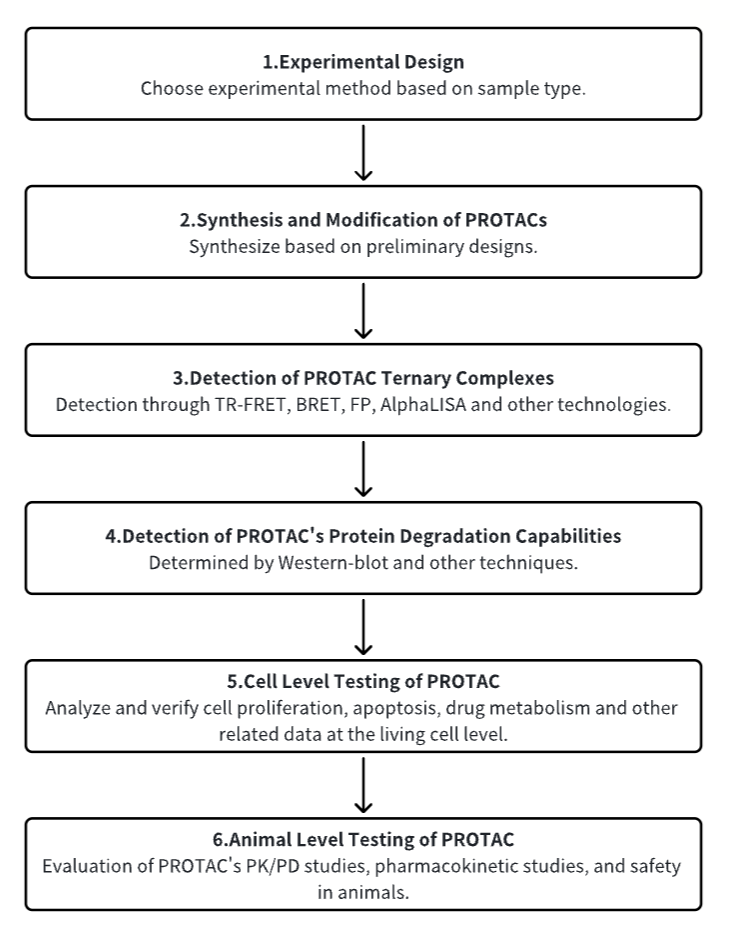
Analysis Workflow
1. Establish the Experimental Workflow Based on Specific Research Needs
2. Synthesize and Modify PROTACs
3. Detect PROTAC Ternary Complexes
4. Detect PROTAC's Protein Degradation Capabilities
5. Test the Cell Level of PROTAC
6. Test the Animal Level of PROTAC
Service Advantages
1. Provide Professional PROTAC Design Consultation to Facilitate Efficient and Rational Drug Design
2. Offer Rapid, Cost-Effective, and High-Quality Synthesis Services
3. Make Available a Commercial Library of PROTACs for Selection
4. Offer Several Advanced Screening Platforms Capable of Thoroughly Analyzing and Testing the Activity of Potential PROTAC Candidates
Sample Results
1. Highly Active and Selective PROTAC Targeting ALK Discovery
Approved small-molecule inhibitors targeting anaplastic lymphoma kinase (ALK) have demonstrated significant efficacy in some subset of cancer patients. However, the numerous ALK mutants or fusion partners exhibit resistance to these drugs, greatly limiting their clinical use. Despite the drug design strategy of PROTAC holds great potential to overcome drug resistance in theory, there are obvious disadvantages for the reported PROTACs that include high molecular weight, long linkers, difficult synthesis routes as well as insufficient evidence in activity for diverse ALK mutants. In this study, we designed and synthesized a miniaturized PROTAC of ALK named AP-1, using minimalist design principles with just two simple ligand chemical units and a two-atom linker. AP-1 effectively degraded various ALK mutant forms, including NPM-ALK, EML4-ALK, and F1174L mutant ALK, showing potent activity and high selectivity in ALK-positive cells. In xenograft mouse models, AP-1 demonstrated superior antitumor efficacy compared to existing treatments like ceritinib and other ALK degraders. AP-1 with an extremely simple PROTAC structure offers an effective treatment option for various ALK-positive cancers and provides valuable insights into overcoming common challenges associated with PROTAC development, such as excessive molecular weight and poor solubility.
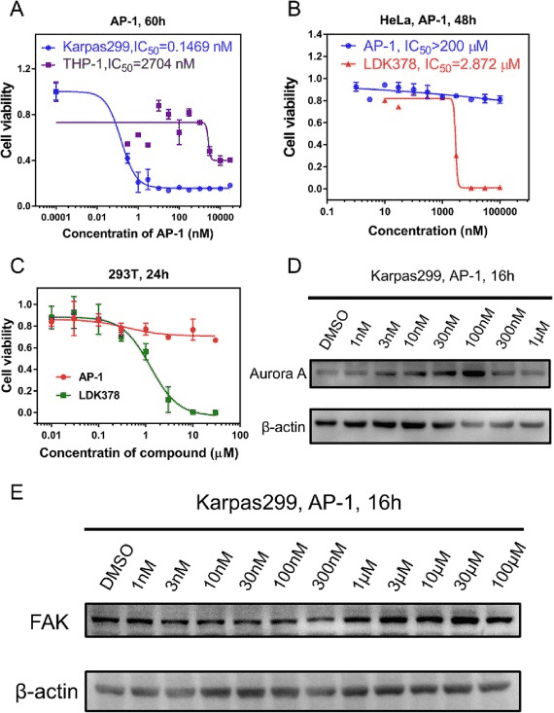
Figure 8. Selectivity, Specificity, and Safety Analysis of AP-1 [9]
2. PROTAC Targeting ERK5 Synthesis and Screening
Extracellular signal-regulated kinase 5 (ERK5) is a crucial enzyme within the mitogen-activated protein kinase family, implicated in tumor growth, migration, and angiogenesis. However, the results of ERK5 inhibition in multiple studies are controversial, and a highly specific ERK5-targeting agent is required to confirm physiological functions. Using PROTAC technology, we designed a selective ERK5 degrader PPM-3, and evaluated its biological effects on cancer cells. Interestingly, the selective degradation of ERK5 with PPM-3 did not directly impact tumor cell growth. Proteomics analyses suggest that ERK5 deficiency may influence tumor immunity. PPM-3 modulates tumor development by affecting macrophage differentiation, making it not only an effective tool for studying ERK5 but also a promising candidate for immunotherapy.
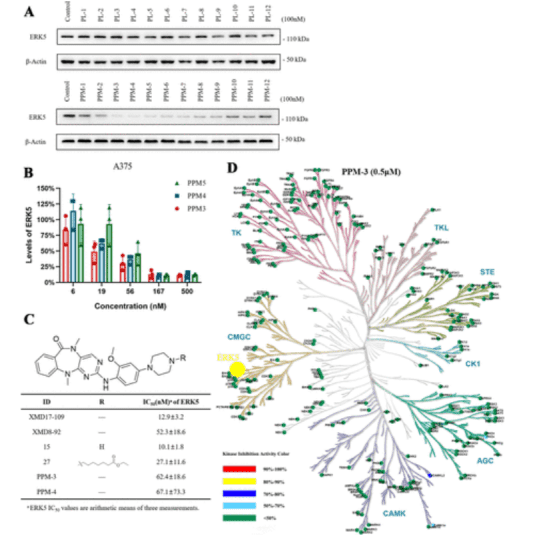
Figure 9. Evaluation of ERK5 Degrader's Efficiency, Kinase Activity, and Selectivity [10]
3. Analyzing PROTACs with Thermal Shift Assay-based MS
Targeted protein degradation is a compelling area of study that offers potential improvements over traditional small-molecule therapeutics in terms of dosage, side effects, resistance, and targeting "undruggable" proteins. A key challenge in designing degraders, such as molecular glues, is that effective and selective protein degradation does not require binding to the POI; instead, it is essential to understand interactions with the responsible ligase. Similarly, for PROTACs, understanding the binding characteristics of the POI alone is not sufficient. Therefore, simultaneously assessing the binding to both POI and the E3 ligase as well as the resulting degradation profile is of great value. The thermal shift assay is an unbiased cell-based method, designed to investigate the interaction of compounds with their cellular protein targets by measuring compound-induced changes in protein thermal stability. In combination with MS, thermal shift assay can simultaneously evaluate compound-induced changes in the stability of thousands of proteins. This method has been employed to analyze a variety of protein degraders, including molecular glues and PROTACs, to elucidate mechanisms of action and deconvolve off-target effects in intact cells. By observing changes in protein thermal stability and simultaneously assessing protein abundance, thermal shift assay-based MS allows us to correlate target engagement (i.e., binding to both POI and ligase) with functional readouts (i.e., protein degradation induced by degraders).
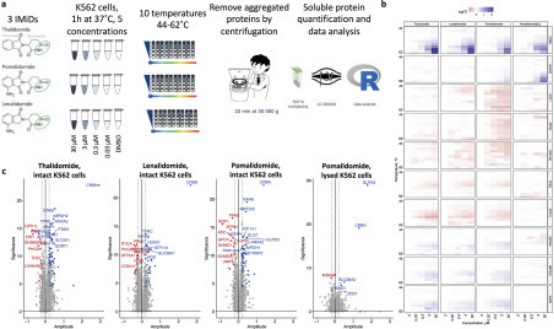
Figure 10. Thermal Shift Assay-based MS Evaluation of PROTAC [11]
Sample Submission Requirements
1. Comprehensive Experimental Steps
2. Specifications of Relevant Instrumentation
3. Compilation of Original Experimental Data
4. Report on the Physicochemical Properties and Efficacy of PROTACs
Applications
1. Pro-PROTAC Development to Address On-Target Off-Tumor Toxicity
PROTACs offer a revolutionary approach to protein degradation, with transformative implications for managing a variety of diseases. Despite notable advantages, the possibility of on-target off-tumor toxicity in healthy cells represents a critical challenge to clinical applications in cancer treatment. Current research focuses on exploring strategies to enhance targeted degradation activity in a cell-selective manner to minimize undesirable side effects. Innovations such as prodrug-based PROTACs (pro-PROTACs), which activate to release their effects primarily within tumor-targeted, are being developed to enhance the clinical utility of this technology.
Figure 11. Pro-PROTAC Reduces Off-Tumor Targeted Toxicity [12]
2. PROTACs for Agriculture: A New Biotechnological Weed Control Solution [13]
Weed control has relied on the use of organic and inorganic molecules that interfere with druggable targets, especially enzymes, for almost a century. While effective, this method has led to widespread herbicide resistance. The rate of discovery of new druggable targets that are selective and with favorable environmental profiles has slowed down, highlighting the need for innovative control tools. The advent of PROTAC technology, originally developed for human diseases, offers potential applications in agriculture by using small bait molecules linked to E3 ligases. The 3-dimensional structure of the bait favors physical interaction with a binding site in the target protein in a manner that allows E3 recruitment, ubiquitination and then proteasome-mediated degradation. This system makes it possible to circumvent the need to find druggable targets because it can degrade structural proteins, transporters, transcription factors, and enzymes without the need to interact with the active site. PROTAC can help control herbicide-resistant weeds as well as expand the number of biochemical targets that can be used for weed control.
3. Emerging Role of PROTACs in Treating AD
Proteolysis-targeting technology is a new emerging technique to target mutated, denatured, and misfolded proteins that accumulate in various parts of the body. The accumulation of these aggregated harmful proteins, such as β-amyloid (Aβ), tau, mHTT, polyglutamates, and other proteins in the brain, are some of the reasons for the development of neurodegenerative diseases. Aβ accumulation and hyperphosphorylation are the common signals for the progression of Alzheimer's disease (AD). The challenge of targeting these proteins with traditional small-molecule inhibitors due to their undruggable nature has been significant. However, the body's inherent protein quality control systems, which remove these aggregates, represent a natural defense mechanism. Advances in medicinal chemistry have led to the development of targeted protein degradation technologies that leverage the UPS to dissolve/degrade misfolded proteins implicated in diseases, offering a new avenue for therapeutic intervention in conditions like AD.
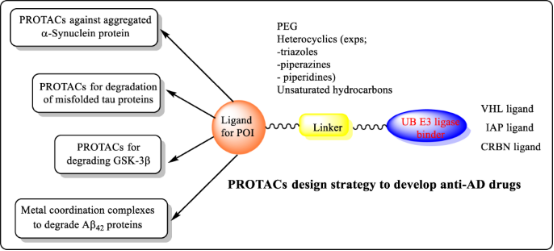
Figure 12. PROTAC Application in Alzheimer's Disease [14]
FAQ
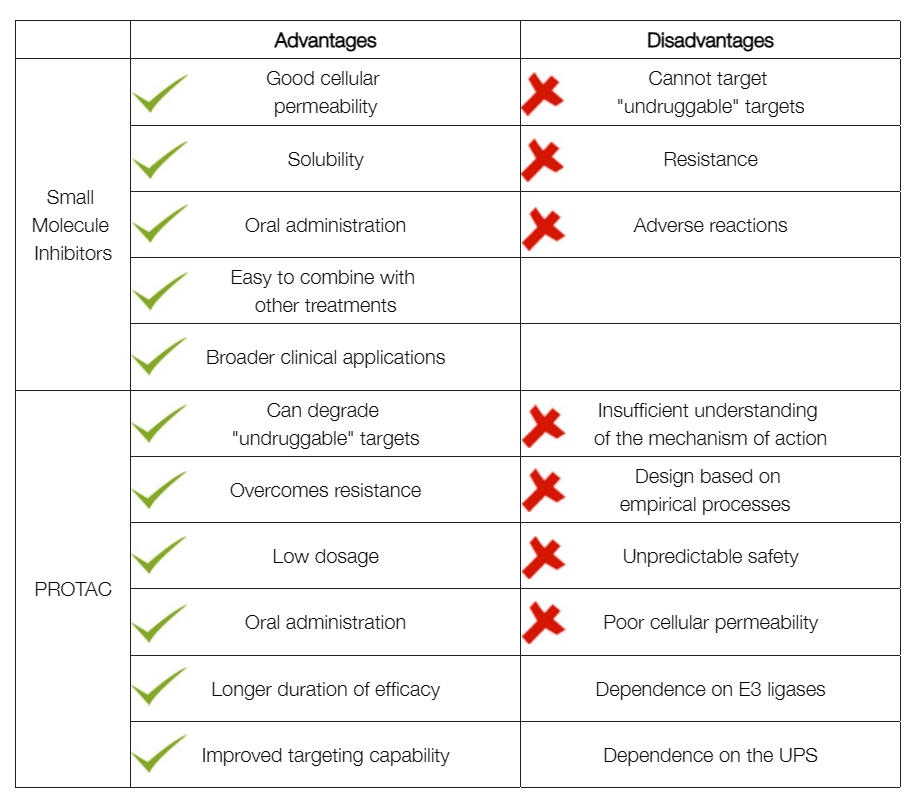
Q1: What are the advantages of PROTACs compared to conventional small-molecule inhibitors?
References
[2] Amirian R, Azadi Badrbani M, Izadi Z, Samadian H, Bahrami G, Sarvari S, Abdolmaleki S, Nabavi SM, Derakhshankhah H, Jaymand M. Targeted protein modification as a paradigm shift in drug discovery. Eur J Med Chem. 2023 Nov 15;260:115765. doi: 10.1016/j.ejmech.2023.115765. Epub 2023 Aug 29. PMID: 37659194.
[3] Fang Y, Wang S, Han S, Zhao Y, Yu C, Liu H, Li N. Targeted protein degrader development for cancer: advances, challenges, and opportunities. Trends Pharmacol Sci. 2023 May;44(5):303-317. doi: 10.1016/j.tips.2023.03.003. PMID: 37059054.
[4] Sincere NI, Anand K, Ashique S, Yang J, You C. PROTACs: Emerging Targeted Protein Degradation Approaches for Advanced Druggable Strategies. Molecules. 2023 May 10;28(10):4014. doi: 10.3390/molecules28104014. PMID: 37241755; PMCID: PMC10224132.
[5] Lin W, Chen T. General Stepwise Approach to Optimize a TR-FRET Assay for Characterizing the BRD/PROTAC/CRBN Ternary Complex. ACS Pharmacol Transl Sci. 2021 Feb 26;4(2):941-952. doi: 10.1021/acsptsci.1c00032. PMID: 33860212; PMCID: PMC8033776.
[6] Sun Y, Yang Z, Zhang Z, Li Z, Guo L, Pan H, Luo X, Liu D, Rao Y. Design, synthesis, and evaluation of BTK-targeting PROTACs with optimized bioavailability in vitro and in vivo. RSC Med Chem. 2023 Jun 21;14(8):1562-1566. doi: 10.1039/d3md00216k. PMID: 37593574; PMCID: PMC10429852.
[7] Zhang AX, Cassidy K, Dahl G, Moreau K, Pachl F, Zuhl AM. The Vital Role of Proteomics in Characterizing Novel Protein Degraders. SLAS Discov. 2021 Apr;26(4):518-523. doi: 10.1177/2472555220985776. Epub 2021 Feb 20. PMID: 33615886.
[8] Pike A, Williamson B, Harlfinger S, Martin S, McGinnity DF. Optimising proteolysis-targeting chimeras (PROTACs) for oral drug delivery: a drug metabolism and pharmacokinetics perspective. Drug Discov Today. 2020 Oct;25(10):1793-1800. doi: 10.1016/j.drudis.2020.07.013. Epub 2020 Jul 18. PMID: 32693163.
[9] Gong L, Li R, Gong J, Ning X, Sun J, Ma Q, Zhu C, Yang Y, Lin K, Li Y, Zhang Q, Li T, Lin Z. Discovery of a miniaturized PROTAC with potent activity and high selectivity. Bioorg Chem. 2023 Jul;136:106556. doi: 10.1016/j.bioorg.2023.106556. Epub 2023 Apr 21. PMID: 37105002.
[10] Pan P, Geng T, Li Z, Ding X, Shi M, Li Y, Wang Y, Shi Y, Wu J, Zhong L, Ji D, Li Z, Meng X. Design, Synthesis, and Biological Evaluation of Proteolysis-Targeting Chimeras as Highly Selective and Efficient Degraders of Extracellular Signal-Regulated Kinase 5. J Med Chem. 2023 Sep 26. doi: 10.1021/acs.jmedchem.3c00864. Epub ahead of print. PMID: 37751283.
[11] Chernobrovkin AL, Cázares-Körner C, Friman T, Caballero IM, Amadio D, Martinez Molina D. A Tale of Two Tails: Efficient Profiling of Protein Degraders by Specific Functional and Target Engagement Readouts. SLAS Discov. 2021 Apr;26(4):534-546. doi: 10.1177/2472555220984372. Epub 2021 Jan 15. PMID: 33445986.
[12] Chen C, Yang Y, Wang Z, Li H, Dong C, Zhang X. Recent Advances in Pro-PROTAC Development to Address On-Target Off-Tumor Toxicity. J Med Chem. 2023 Jul 13;66(13):8428-8440. doi: 10.1021/acs.jmedchem.3c00302. Epub 2023 Jun 14. PMID: 37317568.
[13] Chen C, Yang Y, Wang Z, Li H, Dong C, Zhang X. Recent Advances in Pro-PROTAC Development to Address On-Target Off-Tumor Toxicity. J Med Chem. 2023 Jul 13;66(13):8428-8440. doi: 10.1021/acs.jmedchem.3c00302. Epub 2023 Jun 14. PMID: 37317568.
[14] George, N., Akhtar, M.J., Balushi, K.A. et al. The emerging role of proteolysis targeting chimeras (PROTACs) in the treatment of Alzheimer's disease. Med Chem Res 32, 601–616 (2023). https://doi.org/10.1007/s00044-023-03026-w
How to order?







