Pros and Cons of 3 Common Protein Structure Analysis Methods
Proteins, as fundamental molecules driving biological processes, have their three-dimensional structures directly determining their functions. Accurate analysis of protein structure not only facilitates elucidation of biological mechanisms but also provides critical insights for drug discovery and disease mechanism studies. Currently, the principal techniques for protein structure analysis include X-ray crystallography, nuclear magnetic resonance (NMR) spectroscopy, and cryo-electron microscopy (Cryo-EM).
X-ray Crystallography: The Gold Standard for High-Resolution Structure Determination
X-ray crystallography determines atomic-level three-dimensional structures by analyzing X-ray diffraction patterns generated by protein crystals. This method is particularly valued for its exceptionally high resolution (often below 1.0 Å) and its suitability for high-throughput analysis of numerous samples. Presently, approximately 80% of structures deposited in the Protein Data Bank (PDB) have been resolved using this technique.
1. Advantages
(1) Provides ultra-high resolution, allowing clear visualization of amino acid side chains, metal ions, and water molecules within proteins.
(2) Supported by a mature experimental and computational framework, including established software packages such as Phenix and CCP4.
(3) Broadly applicable to a wide range of crystallizable proteins, including enzymes, receptors, and antibodies.
2. Limitations
(1) Crystallization can be technically challenging, particularly for membrane proteins and proteins with extensive flexible regions.
(2) Produces static structures, as the crystalline environment may constrain the protein’s native conformation and dynamic behavior.
(3) Sample preparation is time-intensive, often requiring weeks to months from crystallization screening to data analysis.
Nuclear Magnetic Resonance (NMR) Spectroscopy: Elucidating Solution-State Structures and Dynamics
NMR spectroscopy exploits resonance signals from nuclear spins in solution and reconstructs protein structures through the analysis of multidimensional spectra. This protein structure analysis smethod is especially valuable for probing flexible regions and dynamic behavior in proteins.
1. Advantages
(1) Offers structural information under near-physiological conditions, providing insights closer to the protein’s native state within cells.
(2) Captures dynamic properties by revealing not only static structures but also conformational changes and motion.
(3) Does not require crystallization, making it well-suited for proteins that are challenging to crystallize, such as intracellular domains of membrane proteins.
2. Limitations
(1) Typically limited to proteins smaller than 50 kDa, as resolving larger complexes remains technically demanding.
(2) Signal complexity and spectral overlap necessitate high sample purity and concentration, along with substantial expertise for data interpretation.
(3) Delivers relatively lower resolution compared to the atomic-level detail achievable by X-ray crystallography.
Cryo-Electron Microscopy (Cryo-EM): A Key Technique for Large Complexes and Dynamic Conformations
Cryo-EM involves rapid sample vitrification, imaging with low-temperature electron beams, and subsequent reconstruction of three-dimensional density maps, making it ideal for analyzing macromolecular complexes. Recent advancements in single-particle imaging and improved resolution, now approaching 2 Å, have positioned Cryo-EM as a leading methodology in structural biology.
1. Advantages
(1) Does not require crystallization, enabling studies of large, challenging complexes such as viral particles, ribosomes, and membrane protein assemblies.
(2) Capable of resolving macromolecular complexes with molecular weights in the megadalton range.
(3) Facilitates analysis of multiple conformational states within a single system, shedding light on functionally relevant dynamics.
(4) The recent resolution revolution in Cryo-EM has elevated its resolving power to a level comparable to X-ray crystallography.
2. Limitations
(1) Demands high-purity, homogeneously dispersed samples, with stringent control over specimen preparation and ice layer thickness.
(2) Requires costly instrumentation, with high-end cryo-electron microscopes (e. g. Titan Krios) and their maintenance posing significant financial barriers.
(3) Involves complex data processing, including image alignment, three-dimensional reconstruction, and heterogeneity classification, all necessitating substantial computational resources and advanced algorithms.
Comparative Analysis and Integrated Application of the Three Techniques
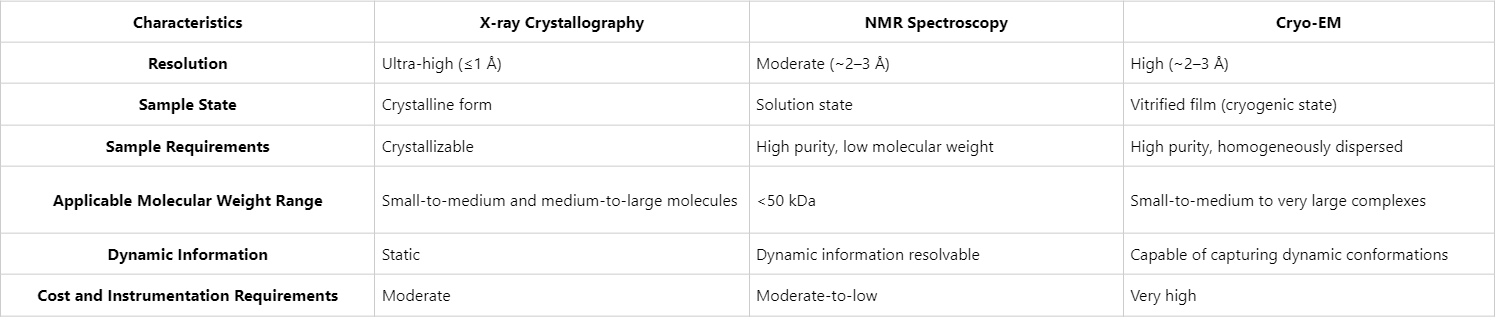
These three protein structure analysis methods are complementary rather than mutually exclusive in practice. X-ray crystallography excels at resolving high-resolution static structures, NMR provides insights into solution-state conformations and dynamics, and Cryo-EM offers unparalleled advantages in characterizing macromolecular assemblies and multiple conformations. Researchers frequently integrate these methods—for instance, resolving the overall architecture of a membrane protein complex via Cryo-EM, refining domain-level resolution using X-ray crystallography, and characterizing flexible regions and dynamic features through NMR.
From resolution and applicability to experimental requirements, X-ray crystallography, NMR spectroscopy, and Cryo-EM each possess distinct strengths and limitations. When selecting an appropriate technique, researchers must consider the molecular characteristics of the target protein (e. g. molecular weight, flexibility, crystallization feasibility), the scientific objectives (high-resolution static structures, dynamic conformations, or complex assemblies), and available resources (instrumentation and time constraints). MtoZ Biolabs offers comprehensive protein structure analysis services, enabling scientists to elucidate the intricate relationship between protein structure and function, thereby accelerating scientific breakthroughs and fostering innovation.
MtoZ Biolabs, an integrated chromatography and mass spectrometry (MS) services provider.
Related Services
How to order?







