Prenylation Proteomics Service
Prenylation is a lipid-based post-translational modification (PTM) involving the covalent attachment of hydrophobic isoprenoid groups such as farnesyl (C15) or geranylgeranyl (C20) to cysteine residues near the C-terminus of target proteins. This modification facilitates membrane association, subcellular localization, protein-protein interactions, and functional regulation of numerous signaling proteins. Key enzymes involved in prenylation include farnesyltransferase (FTase) and geranylgeranyltransferases (GGTase I and II), which recognize specific CaaX motifs (where C is cysteine, a is an aliphatic residue, and X determines enzyme specificity).
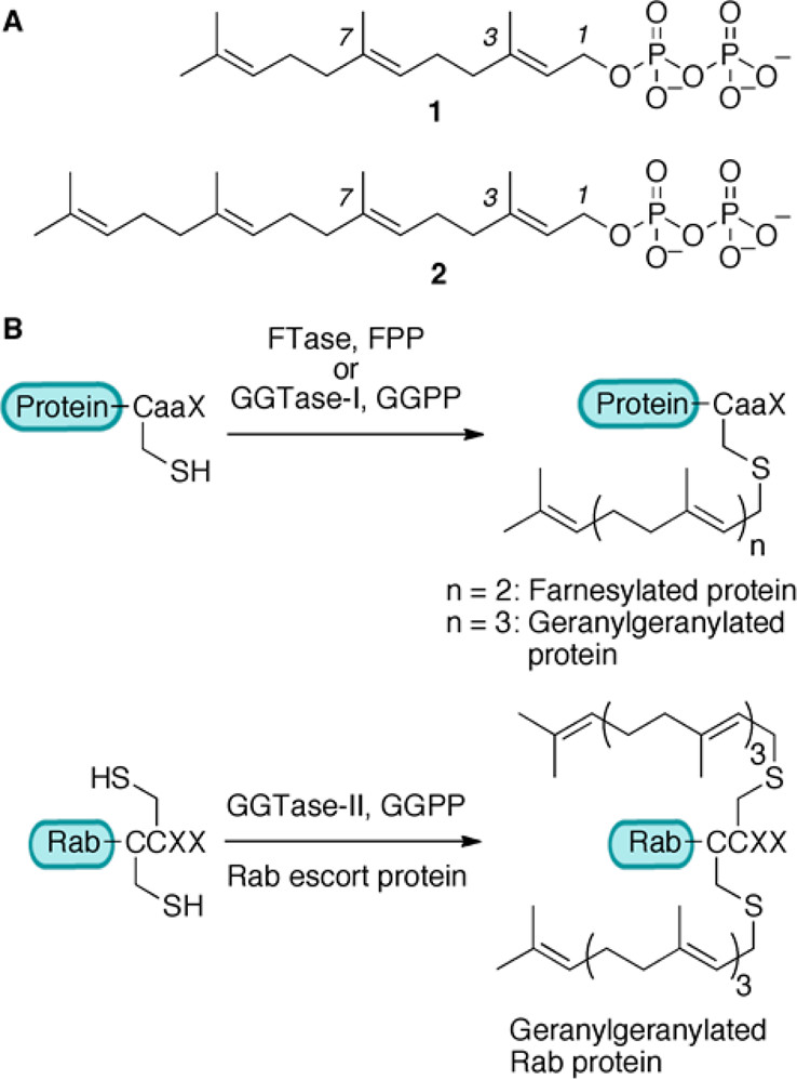
Figure 1. (A) Structures of 1 (Farnesyl Diphosphate, FPP) and 2 (Geranylgeranyl Diphosphate, GGPP)
(B) Reactions Catalyzed by Prenyltransferase Enzymes
Prenylation is essential for the activity of small GTPases such as Rho, many of which are crucial regulators of cell growth, migration, and vesicular trafficking. Dysregulation of prenylation is implicated in various diseases, including cancer, inflammation, and premature aging. Therefore, systematic profiling of prenylation proteomics is vital for understanding disease mechanisms and discovering potential therapeutic targets. However, detecting prenylated proteins remains challenging due to their low abundance, and hydrophobicity. Enrichment strategies combined with advanced mass spectrometry are essential to overcome these limitations.
Service at MtoZ Biolabs
MtoZ Biolabs has launched a specialized Prenylation Proteomics Service that integrates advanced enrichment chemistry with high-resolution mass spectrometry and expert bioinformatics interpretation. This service is designed to support researchers investigating protein lipidation, membrane dynamics, signaling pathways, and disease-related prenylation mechanisms.
Our solution combines bioorthogonal metabolic labeling, enrichment, and state-of-the-art LC-MS/MS platforms to enable highly sensitive and specific identification of prenylated peptides. In addition to qualitative site identification, MtoZ Biolabs offers multiple quantitative proteomics options, including SILAC, TMT labeling, and data-independent acquisition (DIA). These approaches allow clients to assess changes in prenylation across conditions such as drug treatment, gene knockout, or disease progression.
Our Prenylation Proteomics Service delivers a full analytical workflow—from sample preparation and selective enrichment to LC-MS/MS-based peptide identification, quantification, and bioinformatics analysis. Whether you aim to discover prenylated substrates of interest, validate prenyltransferase inhibitors, or explore membrane-associated signaling proteins, MtoZ Biolabs provides a robust and customizable solution to advance your research.
Why Choose MtoZ Biolabs?
✔️Cutting-edge technologies and high-performance platforms for confident prenylation profiling.
✔️Advanced enrichment and detection strategies for low-abundance prenylated peptides.
✔️Experienced team in PTM proteomics analysis.
✔️Customized workflows and technical consultation.
✔️Comprehensive data reporting with in-depth bioinformatics interpretation.
Applications
1. Cancer Research
Characterize prenylation-dependent activation of oncogenic GTPases such as Rho; assess therapeutic response to prenyltransferase inhibitors in tumor models.
2. Infectious Disease and Host Defense
Analyze prenylation of immune-related small GTPases involved in phagocytosis, antigen presentation, and pathogen resistance.
3. Drug Discovery and Target Validation
Evaluate the efficacy and selectivity of farnesyltransferase and geranylgeranyltransferase inhibitors; identify downstream prenylation changes as pharmacodynamic biomarkers.
4. Cell Signaling and Membrane Biology
Study the membrane targeting, localization, and protein–protein interactions driven by prenylation in signaling cascades.
5. Developmental and Stem Cell Biology
Profile dynamic changes in prenylation during cell differentiation, tissue morphogenesis, and organ development.
Case Study
Prenylated PALM2 Promotes the Migration of Esophageal Squamous Cell Carcinoma Cells Through Activating Ezrin
A study identified Paralemmin-2 (PALM2) as a prenylated protein upregulated in esophageal cancer. PALM2 contains a CAAX motif and interacts with farnesyltransferase subunits (FNTA/FNTB), confirming its farnesylation. Mutation of the CAAX site or FTase inhibition disrupted PALM2 membrane localization and impaired its ability to promote cancer cell migration. Mechanistically, prenylated PALM2 activates ezrin by enhancing its membrane targeting and phosphorylation. This study demonstrates how prenylation-dependent signaling regulates tumor progression. MtoZ Biolabs’ Prenylation Proteomics Service enables such discoveries through sensitive identification, quantification, and site mapping of prenylated proteins.
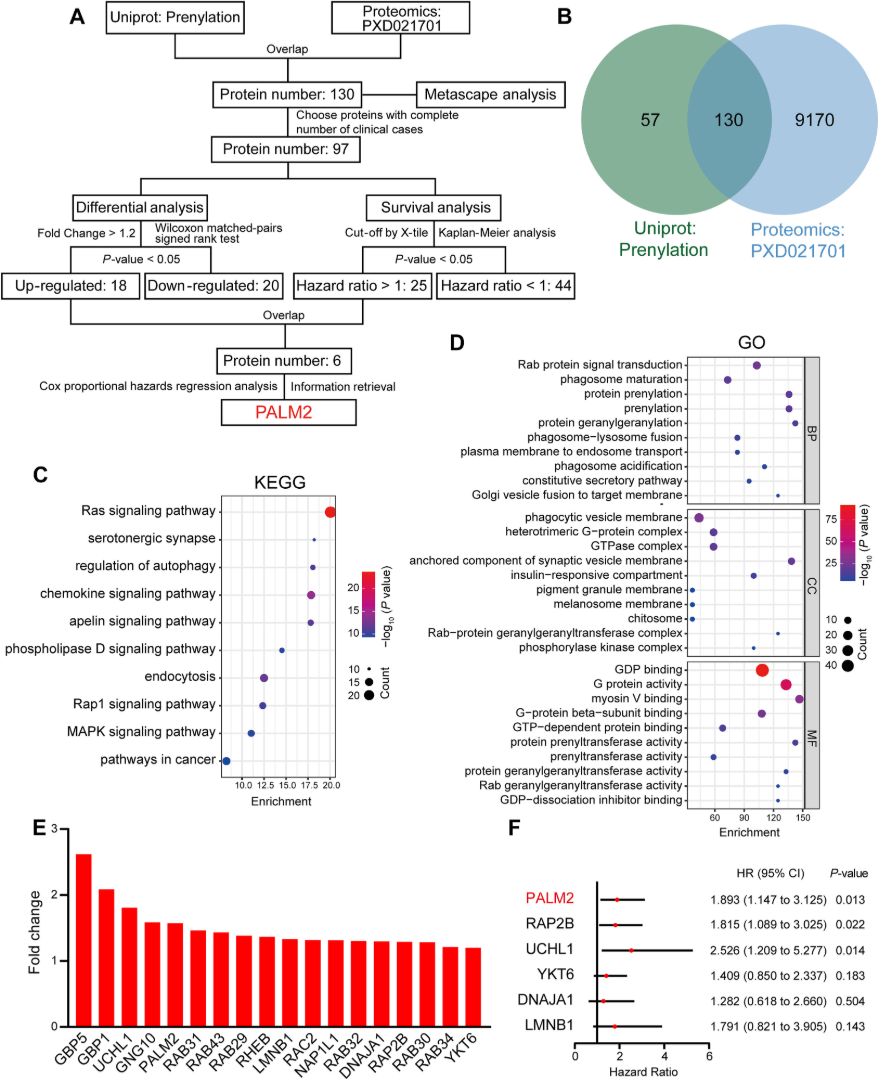
Figure 2. Bioinformatic Analysis of Prenylated Proteins in Esophageal Cancer
FAQ
Q1: What types of samples can be analyzed for prenylation proteomics?
A1: We accept a wide range of sample types, including cell lysates, cultured cell pellets, and tissue samples. Samples should be free of detergents or high concentrations of salts to ensure optimal labeling and enrichment efficiency.
Q2: Do I need to perform metabolic labeling before sending samples?
A2: No. We offer in-house metabolic labeling using alkynyl-farnesol or geranylgeraniol analogs. However, if you have already labeled your samples, please inform us so we can tailor the workflow accordingly.
Q3: Can this service distinguish between farnesylation and geranylgeranylation?
A3: Yes. Depending on the labeling reagent and MS fragmentation patterns, we can differentiate between farnesyl- and geranylgeranyl-modified peptides and report them separately.
Start your prenylation proteomics project with MtoZ Biolabs today. For pricing, sample submission guidelines, or a free consultation, please contact us.
How to order?







