Peptide Synthesis Service
- Drug Development: Providing essential peptide molecules for targeted drug development, particularly in vaccine research, antibody therapies, and related fields.
- Biomarker Screening: Synthesizing specific peptide markers to aid early disease diagnosis and biomarker discovery.
- Protein Interaction Studies: Peptides serve as key tools for exploring protein-protein interactions and their underlying mechanisms.
- Antibody Engineering: Used for antibody screening, specificity optimization, and antibody-peptide conjugation research.
- Basic Research: Providing research tools and materials for studies in cellular signaling, enzyme catalysis, immune responses, and other areas.
- Peptide sequence, molecular weight, and modification details
- Purity and specifications
- Delivery format and storage recommendations
- Structural confirmation and molecular weight verification
- Purity analysis and modification validation
- N-/C-terminal modifications and special amino acid confirmations
- Mass spectrometry and chromatography analysis
- Post-translational modification verification
- Labeling and conjugation efficiency assessment
- Reconstitution and storage conditions
- Usage recommendations and stability evaluation
- Shipping method and special requirements
Peptide synthesis involves chemically linking amino acids in a specific sequence to create peptide chains. Peptides play critical roles in biological systems, participating in processes such as cell signaling, immune responses, and molecular recognition. By synthesizing specific peptides, we can simulate protein-protein interactions, study protein functions, and develop new drugs and vaccines. MtoZ Biolabs offers high-quality peptide synthesis service, including linear peptides, cyclic peptides, modified peptides, labeled peptides, and protein fragments, to meet the diverse needs of scientific research and the pharmaceutical industry, helping to drive innovation and breakthroughs in science.
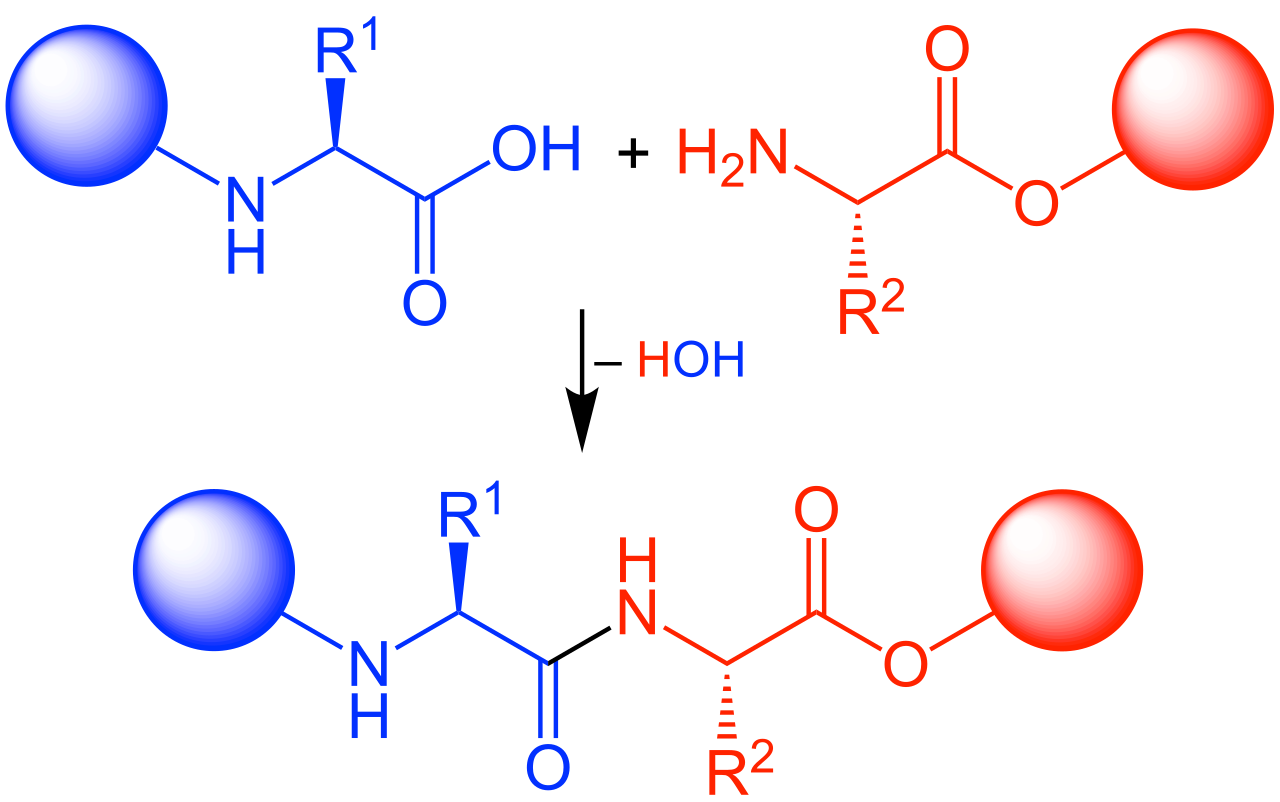
Figure 1. Peptide Synthesis
Technical Principles
Peptide synthesis is primarily carried out using solid phase peptide synthesis (SPPS) technology. SPPS works by sequentially adding amino acids to a solid-phase carrier, where the amino acid’s carboxyl group is activated and reacts with the next amino acid to form a peptide bond. This process typically starts at the C-terminal of the peptide and progresses towards the N-terminal.
Protecting groups play a crucial role in peptide synthesis by preventing unwanted side reactions. Common protecting groups include Fmoc (9-fluorenylmethyloxycarbonyl) and Boc (t-butyloxycarbonyl). Fmoc-based peptide synthesis (Fmoc-SPPS) is widely used due to its efficiency, simplicity, and adaptability.
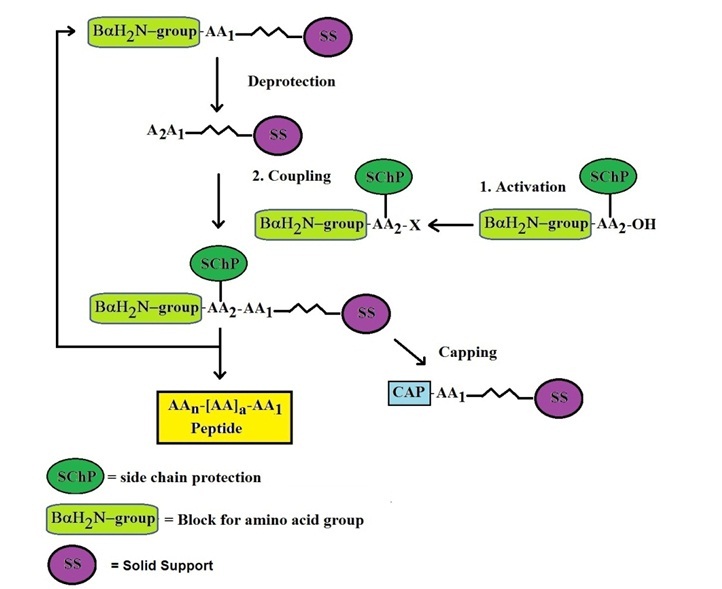
Figure 2. Scheme of SPPS on a Resin as Solid Support with Protected Amino Acids
Services at MtoZ Biolabs
MtoZ Biolabs offers a comprehensive peptide modification and synthesis service that encompasses a wide range of modifications, ranging from basic chemical alterations to complex labeling strategies, which are tailored to meet diverse research needs. With extensive experience and cutting-edge technology, we provide disulfide bond modifications, phosphorylation, isotopic labeling, glycopeptide modifications, biotin and fluorescent labeling, RGD-targeted peptides, polyethylene glycol (PEG) modifications, and protein conjugation to KLH, BSA, and OVA. Additionally, we support custom synthesis of peptides with D-amino acids, amino acid derivatives, and aliphatic carboxylic acids for specialized applications.
For disulfide bond modifications, we precisely construct intra- and intermolecular disulfide bonds, solving the challenge of random disulfide formation in peptides with multiple cysteine residues. Our expertise allows for the incorporation of up to four disulfide pairs in a single peptide chain. In phosphorylation modifications, we have successfully synthesized phosphorylated peptides on serine, threonine, and tyrosine residues, facilitating research on kinase mechanisms. For high-precision quantitative analysis, we offer high-purity isotopically labeled peptides (²H, ¹⁵N, ¹³C, etc.), optimized for MRM and PRM applications. Additionally, we provide a variety of fluorescent labels (such as FITC, Cy series, 5(6)-FAM) and biotin labeling, ensuring high success rates for labeled peptides.
Leveraging state-of-the-art synthesis techniques, rigorous quality control, and a team of skilled professionals, MtoZ Biolabs guarantees that each modified peptide exhibits high purity, stability, and excellent biocompatibility. Whether for fundamental research, drug development, or biomarker discovery, our peptide modification and synthesis services provide reliable support for your scientific endeavors.
Table 1. Peptide Synthesis and Modification Services at MtoZ Biolabs
|
Modification Type |
MtoZ Biolabs Services |
Common Applications |
|
Disulfide Bond Modification |
1. Multi-disulfide bond synthesis |
Enhancing peptide stability, improving protein folding accuracy |
|
Phosphorylation |
1. Phosphorylated peptide synthesis |
Cell signaling pathways, enzyme activity regulation |
|
Isotope Labeling |
¹³C, ¹⁵N, ²H-labeled peptide synthesis |
Mass spectrometry-based quantification, metabolic studies |
|
Methylation |
Mono-, di-, and tri-methylated peptide synthesis |
Epigenetics, protein-protein interactions |
|
Stapled Peptides |
1. Stapled peptide synthesis |
Enhancing α-helix stability, improving cell penetration |
|
Glycopeptides |
1. N-linked glycosylated peptide synthesis |
Immunological studies, vaccine development |
|
Biotin and Fluorescent Labeling |
1. Biotin-labeled peptide synthesis |
Affinity purification, cell imaging, protein interaction studies |
|
RGD Targeting Peptides |
1. RGD sequence peptide synthesis |
Cell adhesion, tumor targeting |
|
Carrier Protein Conjugation |
KLH, BSA, OVA-conjugated antigen peptide synthesis |
Immunological research, antigen preparation |
|
PEGylation |
Linear and branched PEGylated peptide synthesis |
Long-acting drug development, enhanced biological stability |
|
N-Terminal Modification |
1. Acetylation modification |
N-terminal protection, stability enhancement |
|
C-Terminal Modification |
1. C-terminal amidation |
C-terminal protection, improved biological performance |
|
Modified Amino Acids |
1. D-amino acids |
Enhanced bioactivity, membrane permeability |
Service Advantages
1. Advanced Automated Synthesis Platforms
Utilizing state-of-the-art solid-phase peptide synthesis (SPPS) and liquid-phase synthesis (LPPS) techniques, we streamline the production process for fast turnaround times without compromising accuracy.
2. High-Purity Peptide Production
Optimized synthesis strategies and stringent purification processes (HPLC, LC-MS) allow us to achieve purity levels exceeding 95%, reducing the need for further processing.
3. Optimized Protocols for Complex Peptides
Our expertise in handling long peptides, cyclic peptides, multiple disulfide bonds, and post-translational modifications ensures high efficiency in synthesizing structurally complex peptides with minimal side reactions.
4. Accurate Modification and Labeling
We offer precisely controlled modifications such as phosphorylation, glycosylation, methylation, PEGylation, biotinylation, and isotope labeling, with validated site-specific incorporation and modification efficiency assessments.
5. Reproducibility and Scalability
Our validated synthesis protocols ensure batch-to-batch consistency, while scalable production capabilities enable seamless transition from small-scale research peptides to large-scale manufacturing.
6. Fast Turnaround with Rigorous Quality Control
Leveraging high-throughput synthesis systems and an integrated quality control pipeline (MALDI-TOF MS, ESI-MS, HPLC), we deliver accurate and fully characterized peptides in the shortest possible time.
Applications
Peptide synthesis technology has broad applications across various fields, and MtoZ Biolabs' peptide synthesis service support research and development in the following areas:
FAQ
Q1: How do you ensure the quality of peptide synthesis?
We strictly follow standard operating procedures during peptide synthesis, with quality control checks at each step. These checks include verifying the accuracy of the amino acid sequence, the purity of the peptide chain, and the yield and biological activity of the final product.
Q2: What impurities might be present in the peptide?
During peptide synthesis, impurities may include unreacted amino acids, incomplete peptide chains, and by-products formed during the synthesis process. We use efficient purification techniques (such as HPLC) to remove these impurities and ensure that the final peptide product has high purity.
Q3: Why perform N-terminal acetylation and C-terminal amidation modifications?
Chemically synthesized peptides typically have free amino and carboxyl groups at the N- and C-termini. To more closely mimic the natural state of peptides in the parent protein sequence, the peptide ends are often capped through N-terminal acetylation and C-terminal amidation. These modifications help reduce the peptide's net charge, lower its solubility, and better simulate the original state of the α-amino and carboxyl groups in the parent protein.
Deliverables
1. General Information
2. Quality Control and Analysis
3. Data Support
4. Delivery and Usage Guidelines
MtoZ Biolabs offers reliable peptide synthesis service to support your research and development goals. With our advanced SPPS technology, we ensure high-quality peptides tailored to your specifications. Our solutions are designed to facilitate progress in drug development, molecular studies, and biotechnology. Partner with us to advance your scientific innovations and achieve research success. Contact us today to learn more about how we can support your peptide synthesis needs.
MtoZ Biolabs, an integrated chromatography and mass spectrometry (MS) services provider.
Related Services
Cyclic Peptide Synthesis Service
How to order?







