Particle Titer Determination Service | Cryo-EM
- Lipid Nanoparticles (LNPs): Quantifying loaded vs. empty particles for mRNA vaccine formulation.
- Viral Vectors (e.g., AAV, lentivirus): Assessing particle integrity and packaging efficiency.
- Virus-Like Particles (VLPs): Supporting vaccine production with visual confirmation of structural fidelity.
- Extracellular Vesicles (EVs): Measuring particle concentration and morphology in exosome-based diagnostics and therapeutics.
- Gene and RNA Therapeutics: Ensuring reproducible dose delivery in nucleic acid-based drug products.
- Stability Testing: Monitoring aggregation or degradation over time or under stress conditions.
Accurate particle titer determination is essential in the development, quality control, and clinical translation of nanoparticle-based therapeutics and delivery systems. In particular, advanced therapeutic carriers such as lipid nanoparticles (LNPs), virus-like particles (VLPs), viral vectors, and extracellular vesicles (EVs) require precise particle quantification to ensure efficacy, safety, and dose consistency.
Cryo-EM is a label-free, direct imaging technique that freezes nanoparticles in a thin layer of vitreous ice, preserving morphology without chemical fixation. The sample is rapidly plunge-frozen and transferred to a high-resolution transmission electron microscope operating at cryogenic temperatures.
Unlike light-based methods, Cryo-EM allows for:
✔️Direct particle counting per field of view;
✔️Differentiation between empty and full particles;
✔️Identification of aggregates or malformed structures;
✔️Measurement of particle integrity, lamellarity, and membrane composition.
By acquiring multiple fields across different regions of the grid, and applying statistical analysis to the captured images, a reliable estimate of particle titer (particles per mL) is achieved with high confidence and visual traceability.
Service at MtoZ Biolabs
MtoZ Biolabs offers a high-precision Particle Titer Determination Service based on Cryo-EM. Our Particle Titer Determination Service provides end-to-end imaging and analysis to support nanoparticle quantification in vaccine development, gene therapy, drug delivery, and exosome research. Our service is ideal for developers of lipid nanoparticles (LNPs), virus-like particles (VLPs), adeno-associated viruses (AAVs), messenger RNA (mRNA) formulations, and other nanoparticle-based drug delivery systems that require reliable and quantitative characterization. Contact us to explore tailored solutions for your research. Our technical specialists are available to provide a free business assessment.
Analysis Workflow
1. Sample–Bead Mixture Preparation
The sample is diluted and mixed with polystyrene beads of a precisely known concentration. This internal standard provides a reference framework for estimating particle numbers.
2. Grid Staining and Image Acquisition
The mixture is applied to TEM grids and stained using a negative stain (e.g., uranyl acetate). High-quality micrographs are acquired across multiple fields of view to capture both target particles and reference beads.
3. Manual Particle Identification and Annotation
An experienced analyst manually distinguishes and counts both the sample particles and polystyrene beads in each image. Scoring continues until a statistically sufficient number of target particles has been analyzed.
4. Ratio Calculation and Concentration Estimation
The relative abundance of particles versus beads is computed, and using the known bead concentration, the particle titer is extrapolated and reported in particles per mL.
5. Data Reporting and Interpretation
The final report includes the calculated concentration, annotated representative images, and details of other information.
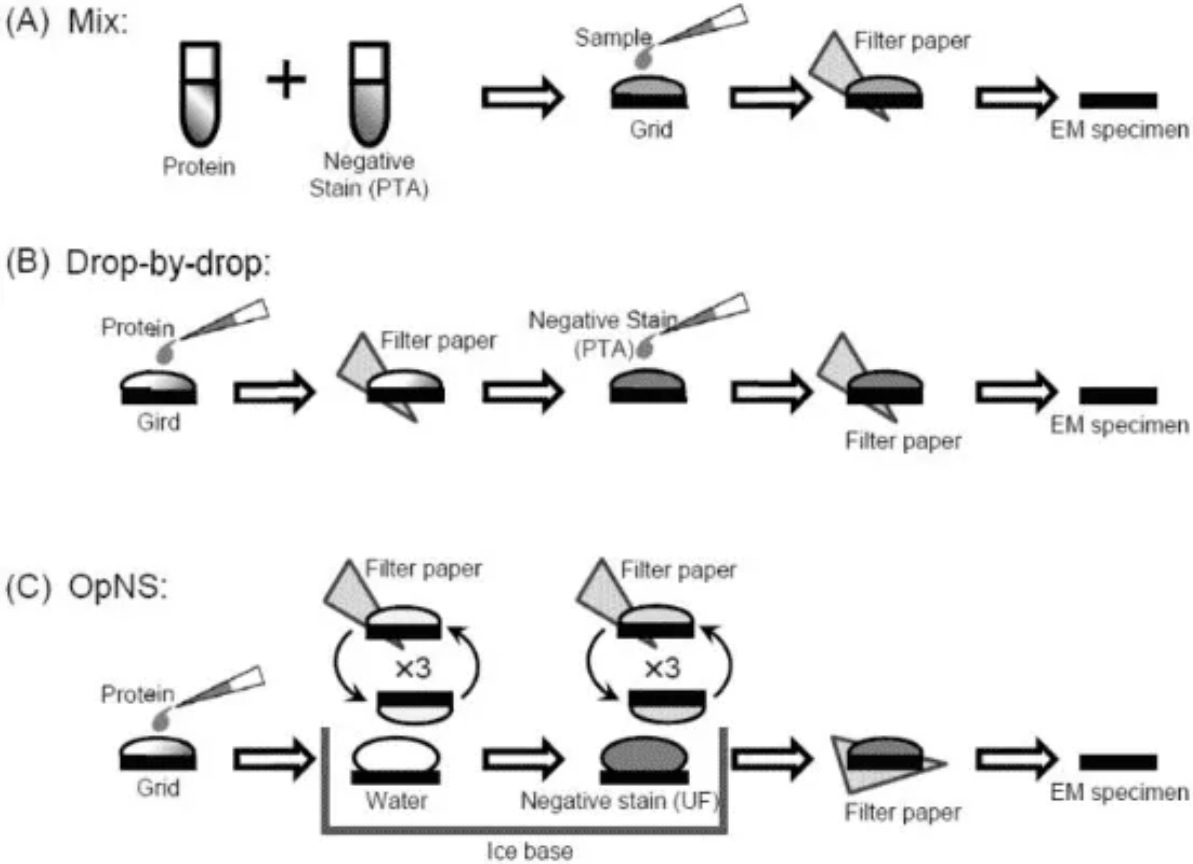
Figure 1. Common Negative Dyeing Processes
Why Choose MtoZ Biolabs?
☑️Direct, label-free quantification of particle number and morphology
☑️Customized protocols for diverse nanoformulation types
☑️High-resolution imaging to differentiate intact, empty, or aggregated particles
☑️Rapid turnaround and responsive technical support
☑️Clear, publication-ready deliverables including annotated micrographs and statistical summaries
Applications
Particle Titer Determination Service based on Cryo-EM provides critical value in the following areas:
FAQ
Q1: How does Cryo-EM titer quantification compare with nanoparticle tracking analysis (NTA) or DLS?
Unlike NTA or DLS, which rely on light scattering and statistical estimation, Cryo-EM offers direct, image-based particle enumeration at nanometer resolution. It enables visual assessment of particle integrity, morphology, and heterogeneity, making it especially suitable for complex or polydisperse formulations.
Q2: Can the analysis distinguish between single particles and aggregates?
Yes. Cryo-EM provides high-resolution images that clearly differentiate monodisperse particles from aggregates or fused structures. This is particularly important for assessing formulation stability and batch-to-batch consistency.
How to order?







