N-myristoylation Proteomics Service
N-myristoylation proteomics analysis is a proteomics technique focused on analyzing N-terminal myristoylation modifications of proteins. Based on high-resolution mass spectrometry platforms and specific enrichment strategies, it enables the high-sensitivity identification of modified proteins and their N-terminal modification sites, providing high-confidence modification spectra and sequence information. N-myristoylation is a co-translational lipid modification, referring to the covalent attachment of myristic acid via an amide bond to a glycine residue after the removal of the initiator methionine. It plays a critical role in regulating protein membrane association, localization, stability, and signal transduction.
N-myristoylation proteomics service is widely applied in studies of membrane protein function, signaling pathway mechanisms, apoptosis and immune regulation, pathogen protein modification analysis, as well as target screening and drug development related to lipid modifications. It is particularly suitable for uncovering the molecular mechanisms of lipid modifications in the onset and progression of diseases. This service, powered by advanced platforms and optimized workflows, provides researchers with comprehensive and reliable solutions for analyzing N-terminal lipid modifications.
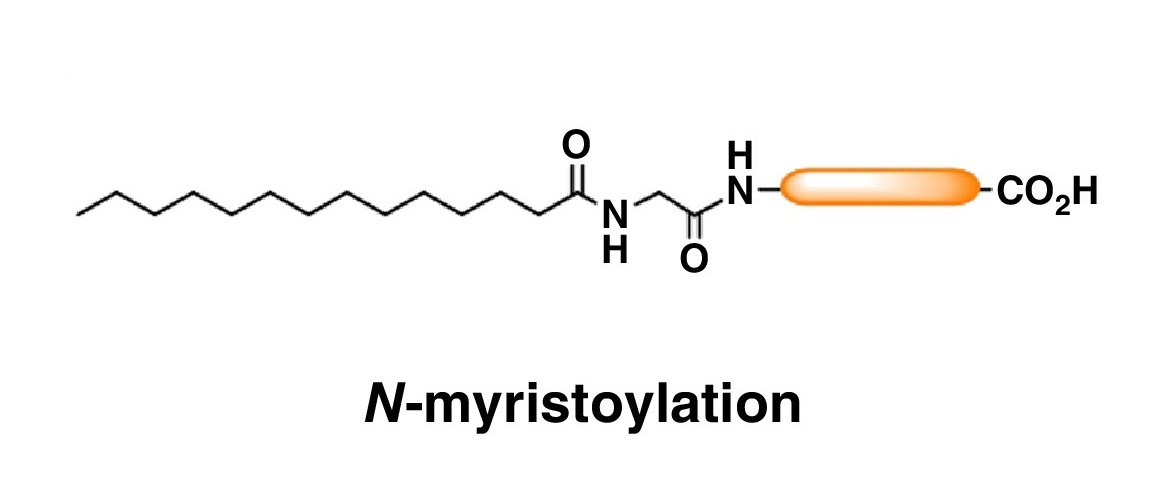
Peng, T. et al. Current Opinion in Chemical Biology, 2016.
Figure 1. N-myristoylation Structural Diagram.
Services at MtoZ Biolabs
Based on high-resolution mass spectrometry platforms, MtoZ Biolabs offers an N-myristoylation proteomics service dedicated to the precise detection and characterization of N-terminal myristoylation modifications in proteins. This service integrates efficient N-terminal peptide enrichment techniques with optimized sample preparation workflows to enable systematic analysis of N-myristoylated proteins in complex samples, accurately identifying modification sites and their corresponding peptide sequences. It provides high-confidence information on modification sites, peptide sequences, and modification spectra, supporting comprehensive analysis and functional research of lipid-modified proteins.
Analysis Workflow
1. Protein Extraction and Digestion
Proteins are extracted from cell, tissue, or biofluid samples, followed by reduction, alkylation, and enzymatic digestion, with preservation of N-terminal information.
2. N-terminal Peptide Enrichment
Specific chemical labeling and selective capture strategies are employed to enrich N-terminal peptides, enhancing the detection efficiency of myristoylated peptides.
3. High-Resolution Mass Spectrometry Analysis
Enriched samples are analyzed with high sensitivity using advanced LC-MS/MS platforms such as the Orbitrap Fusion Lumos.
4. Data Interpretation and Modification Identification
Databases and custom algorithms are used to accurately identify types and specific sites of N-terminal myristoylation modifications.
5. Results Compilation and Report Delivery
Modification spectra, site-specific peptide sequences, and functional annotations are provided to support downstream mechanistic studies and biological interpretation.
Sample Submission Suggestions
1. Sample Types
Compatible with samples from cells, tissues, serum/plasma, body fluids, and purified proteins.
2. Buffer Requirements
Samples should avoid high concentrations of salts, SDS, glycerol, or other components that may interfere with mass spectrometry analysis.
3. Sample Transport
Samples must be stored at -80°C and transported on dry ice to ensure the stability of protein modifications and the integrity of the samples.
Service Advantages
1. Specialized in Lipid Modification Analysis
Focused on the identification and localization of N-terminal myristoylation with dedicated sample processing and data analysis workflows to enhance analytical accuracy.
2. Supported by High-Resolution Platforms
Powered by advanced mass spectrometry platforms such as the Orbitrap Fusion Lumos and integrated with quantitative strategies for comprehensive detection of modification sites and levels.
3. Efficient and Compatible Enrichment Workflow
Utilizes optimized N-terminal enrichment methods compatible with complex sample matrices to improve detection efficiency of low-abundance modified peptides.
4. One-Stop Customized Service
Offers end-to-end customized solutions covering sample preparation, enrichment, mass spectrometry analysis, and data annotation to support functional studies and target discovery.
Applications
1. Membrane Protein Localization Mechanism Studies
N-myristoylation proteomics service can be used to elucidate the role of N-terminal myristoylation in protein membrane association and subcellular localization, revealing regulatory mechanisms of lipid modifications.
2. Signal Transduction Pathway Analysis
By investigating activity regulation mediated by myristoylation of signaling molecules, the service helps uncover functional changes at key signaling nodes.
3. Pathogen–Host Interaction Research
N-myristoylation proteomics service enables the identification of N-terminal modifications in viral or bacterial proteins, shedding light on their roles in infection and immune evasion.
4. Target Discovery and Drug Development
By screening for disease-associated myristoylated proteins, the service supports the development of novel therapeutic targets and lipid modification-based intervention strategies.
Case Study
1. Protein N-Myristoylation: Functions and Mechanisms in Control of Innate Immunity
This study aims to systematically elucidate the functional roles and molecular mechanisms of protein N-terminal myristoylation (N-myristoylation) in the regulation of innate immunity, focusing on a range of immunity-related proteins. The authors analyzed the involvement of N-myristoylation in pattern recognition receptor signaling, activation of inflammatory responses, membrane localization of immune cells, and pathogen clearance. The study reveals that N-myristoylation not only influences the membrane association and spatial distribution of key immune proteins but also participates in maintaining immune homeostasis by modulating the activation status and duration of signaling pathways. Furthermore, viruses can evade immune surveillance by manipulating the N-myristoylation of host proteins. The findings underscore that N-myristoylation is an indispensable lipid modification in regulating innate immune responses, and a deeper understanding of its regulatory network could facilitate the development of novel anti-infective and immunotherapeutic strategies.
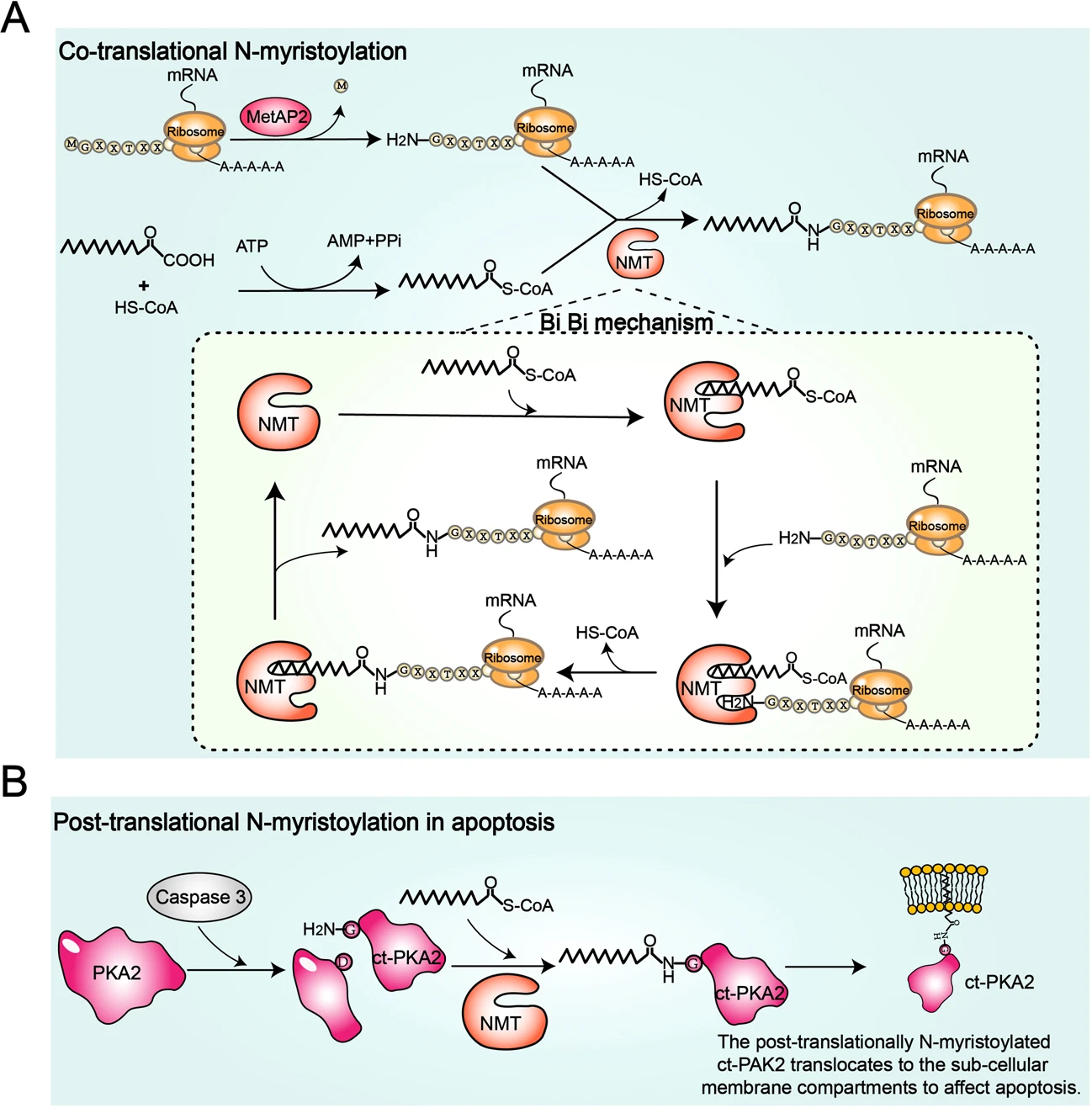
Wang, B. et al. Cellular & Molecular Immunology, 2021.
Figure 2. The Molecular Mechanism of Protein N-Myristoylation Catalyzed by N-Myristoyltransferase (NMT).
2. Global Analysis of N-terminal Myristoylation Modification Using a New Method
This study aims to achieve a global analysis and systematic characterization of the heterogeneity of N-terminal myristoylation modifications, focusing on endogenous proteins in human cells. The research team employed a highly selective and sensitive strategy for the enrichment and identification of N-myristoylated peptides. Coupled with mass spectrometry analysis, this method successfully identified a large number of N-terminal myristoylation sites and revealed modification heterogeneity, including variations among different proteins and multiple modification forms within the same protein. The results demonstrate that N-myristoylation plays a critical regulatory role in various biological processes, such as cell signaling, cytoskeletal organization, and membrane localization. The study concludes that this approach provides an effective tool for comprehensive profiling of N-terminal lipid modifications and contributes to a deeper understanding of their biological significance in cellular functions and disease mechanisms.
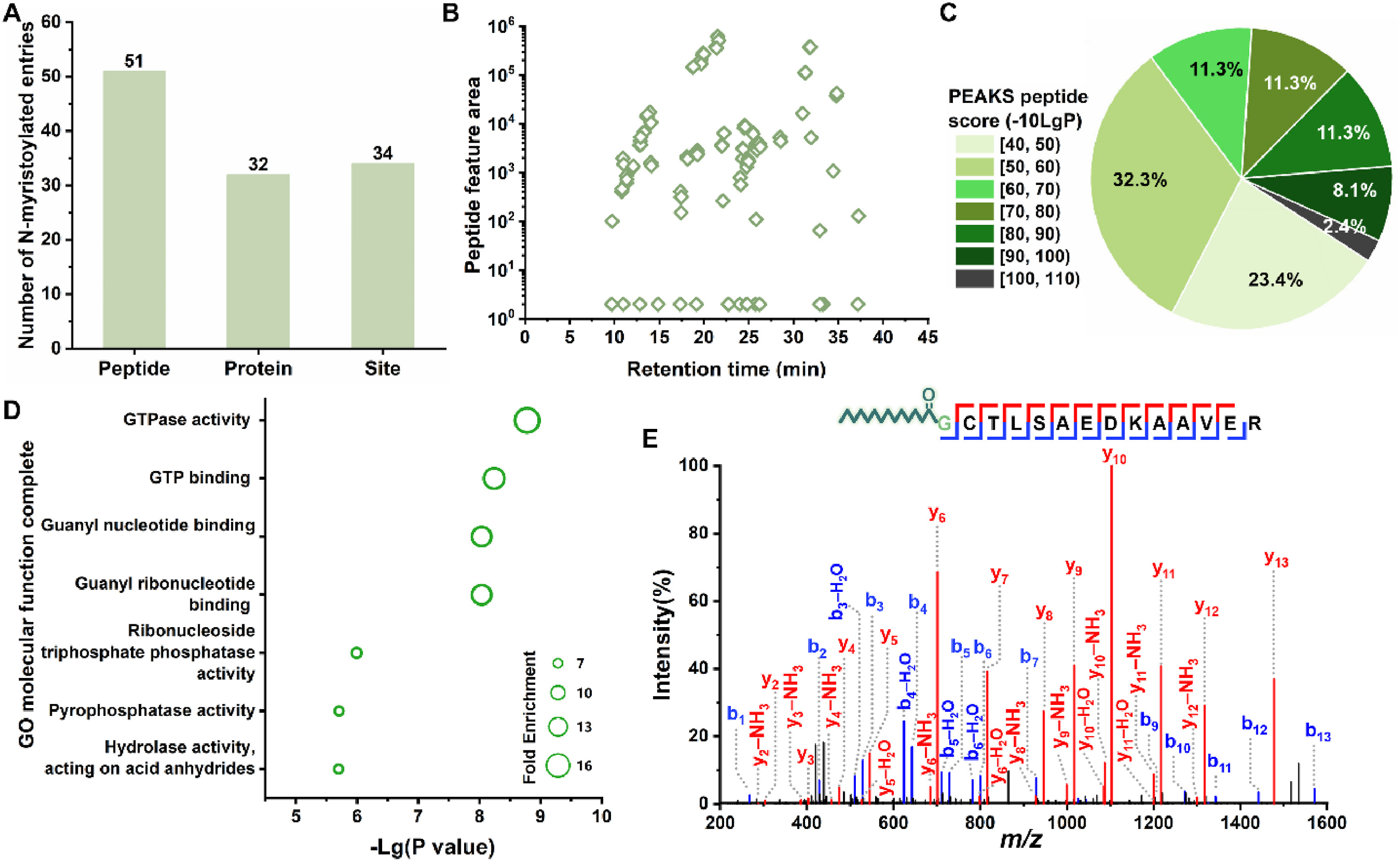
Ji, G H. et al. Talanta, 2024.
Figure 3. N-Myristoylome Profiling with NeS-nGF SPE Method.
FAQ
Q1: What Types of Modifications Can this Service Identify?
A1: This service focuses on detecting N-terminal myristoylation modifications of proteins. It can accurately identify the modification site (Gly residue) and its corresponding peptide sequence, and assess changes in modification levels under different conditions through quantitative strategies.
Q2: Does the Service Support Analysis of Low-Abundance Samples?
A2: Yes. By utilizing an efficient N-terminal enrichment strategy and an ultra-sensitive mass spectrometry platform, we can effectively identify modified peptides even in low-abundance backgrounds. For projects with limited sample amounts, we also offer small-scale pre-analysis evaluation services.
Deliverables
1. Comprehensive Experimental Details
2. Materials, Instruments, and Methods
3. Data Analysis, Preprocessing, and Estimation
4. Bioinformatics Analysis
5. Raw Data Files
How to order?







