N-Glycan Profiling Service | N-Glycan Analysis
As the pharmaceutical industry evolves, biopharmaceutical characterization has gradually become a hot research within the pharmaceutical sector. Glycosylation significantly influences the efficacy, stability, and immunogenicity of protein dru, and the investigation of glycan structures plays a pivotal role in biopharmaceutical characterization processes. N-glycan, a crucial carbohydrate, plays a pivotal role in various cellular processes, including protein folding and signal transduction. Concurrently, it frequently associates with proteins and lipid compounds, yielding glycoproteins and glycolipids. These glycoproteins, commonly found on the cell surface, are instrumental in the recognition of bacteria, viruses, and other proteins, including lectins, a critical aspect in the characterization of biopharmaceuticals. Glucotype is a pharmaceutical consideration in establishing and improving quality standards, the evaluation of glucotype comparability before and after process change, and the similarity evaluation of glucotype of monoclonal antibody biosimilars.
N-glycan is an oligosaccharide structure containing Man3-GlcNAc2, which is linked to the polypeptide chain of a protein by a glycosidic bond between the terminal N-acetylglucosamine (GlcNAc) and the NH2 group of asparagine. Currently, N-glycan analysis predominantly relies on the N-glycosylation profiling of intact glycopeptides. Additionally, free level N-glycan profiling can be achieved through 2AB-UPLC or 2AA-UPLC labeling, facilitated by the action of PNGase F on the N-glycan chain. Sample preparation steps for most glycan analyses include digestion, deglycosylation, purification, labeling/derivatization, and SPE of glycoproteins. The sample's characteristics may necessitate additional steps and techniques in its preparation. MtoZ Biolabs used method based on 2AB-UPLC-MS to detect and analyze the structure of N-glycan chains.
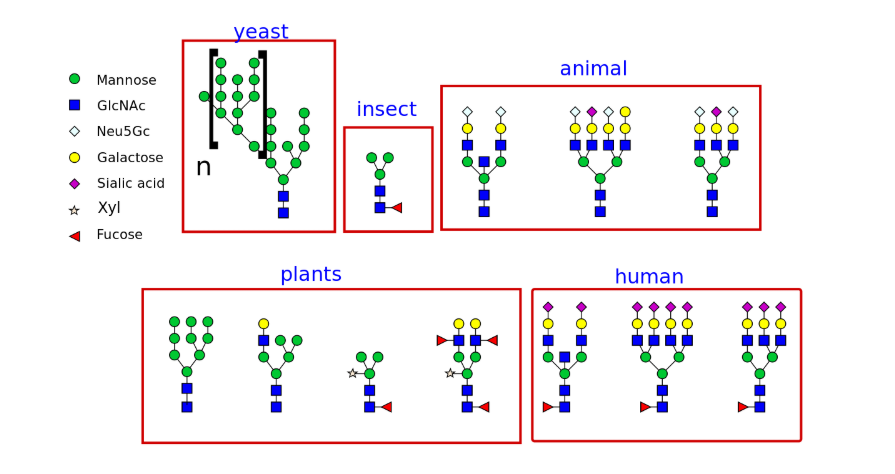
Different types of N-glycans produced in different organisms:
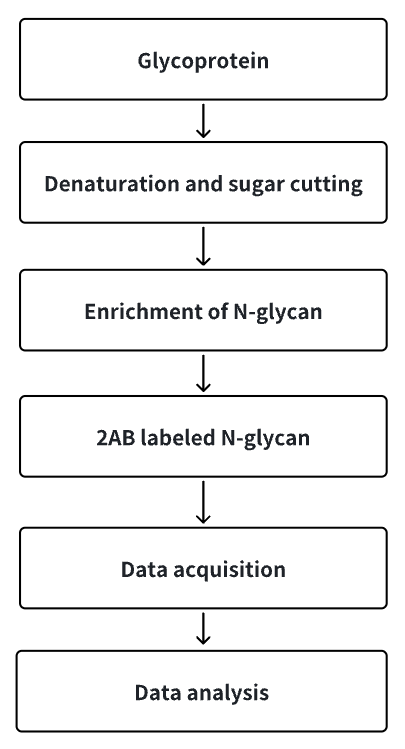
Analysis Workflow
1. Release glycoprotein N-glycan chains.
2. Enrich N-glycan chains.
3. Label N-glycan chains with 2AB reagent.
4. Collect and analyze data.
Technical Principles
Services Advantages
1. Hydrophilic interaction chromatography (HILIC) can be used for N-glycan analysis.
2. N-glycan released by the enzymatic reaction of PNGase F.
3. 2-Aminobenzamide (2-AB) is utilized to derivatize released glycan chains, enabling the analysis of a large array of N-glycan samples.
4. To remove detergents, non-volatile salts, or free amino groups that may interfere with derivatization, N-glycans can be purified using TSK Amide-80 chromatography prior to fluorescent labeling.
5. The labeled N-glycan were analyzed by HILIC-UHPLC MS.
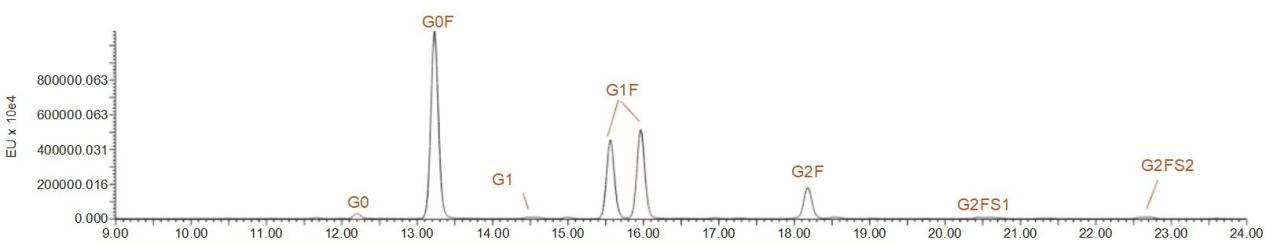
Sample Results
Sample Submission Requirements
1. Monoclonal Antibody
2. Analysis of N-Glycan in Complex Samples
Deliverables
1. Project Report (Including: Experimental Protocols, Relevant Mass Spectrometry Parameters, N-Glycan Details, Mass Spectrometry Pictures, etc.)
2. Raw Data
Applications
It is of great significance in the field of scientific research and clinical medicine.
FAQ
Q1: What are the characteristics of PNGase F enzyme in cleaving N-glycan?
PNGase F is an amidase that cleaves N-linked glycans on glycoproteins. The types of glycans it cleaves include high mannose, hybrid and complex oligosaccharides; the cleavage site is the glycosidic bond between the innermost N-acetylglucosamine and asparagine.
Q2: Does PNGase F cleave all N-glycan?
No, like α1-3 fucose attached to the core GlcNAc residue, it cannot be cleaved, and PNGase FA enzyme can compensate for this glycoform cleavage.
How to order?







