mRNA HPLC Analysis Services
mRNA HPLC analysis is a detection technique based on high-performance liquid chromatography (HPLC), designed for high-resolution separation and quantitative analysis of the purity, integrity, and relevant modifications of messenger RNA (mRNA). This method utilizes the efficient separation capability of HPLC, employing different types of chromatography columns (such as reversed-phase, ion exchange, or hydrophilic interaction chromatography) combined with various detection techniques, including ultraviolet detection (UV), fluorescence detection (FLD), or mass spectrometry (MS), to achieve precise characterization of mRNA samples, ensuring quality control in compliance with the requirements of biopharmaceutical research and production.
mRNA HPLC analysis services are widely applied in the development and quality control of mRNA vaccines and gene therapy drugs, including mRNA synthesis and modification verification, purity assessment, degradation product detection, and translation efficiency optimization. Particularly in the field of mRNA-based therapeutics, this technique can be used to evaluate mRNA stability, identify the impact of different modified nucleotides, and optimize production processes to ensure the safety and efficacy of mRNA drugs, providing critical support for the development of novel biopharmaceuticals.
Services at Mtoz Biolabs
Utilizing a high-performance liquid chromatography (HPLC) platform, Mtoz Biolabs provides mRNA HPLC analysis services that enable high-resolution separation and precise quantitative analysis of mRNA samples. Our services cover all stages from in vitro transcription (IVT) to the purification process, offering high-sensitivity and high-resolution analytical support to ensure a comprehensive assessment of mRNA purity, integrity, and modifications.
Analysis Workflow
1. Sample Preparation
Clients provide mRNA samples, which are appropriately purified and adjusted in concentration according to experimental requirements to ensure optimal detection performance.
2. Chromatographic Method Optimization
Based on the characteristics of the mRNA, a suitable HPLC method (such as reversed-phase, ion exchange, or hydrophilic interaction chromatography) is selected, and parameters including mobile phase, column temperature, and gradient conditions are optimized to achieve efficient separation.
3. HPLC Detection
High-performance liquid chromatography (HPLC/UPLC) is used in combination with ultraviolet detection (UV), fluorescence detection (FLD), or mass spectrometry (MS) to analyze the purity, integrity, and modifications of mRNA.
4. Data Analysis
Quantitative and qualitative analysis of mRNA samples is performed based on parameters such as peak area, retention time, and mass spectrometric interpretation, enabling the identification of potential degradation products, impurities, and structural variations.
5. Results Reporting
A detailed analytical report is provided, including mRNA purity, integrity, degradation characteristics, and modifications. Customized data interpretation is available upon request to support process optimization and quality control.
Service Advantages
1. High-Resolution Detection
Utilizing UPLC/UHPLC systems combined with optimized chromatographic separation methods, we achieve precise separation and quantitative analysis of mRNA, ensuring accurate assessment of purity and integrity.
2. One-Time-Charge
Our pricing is transparent, no hidden fees or additional costs.
3. High Sensitivity and High Reproducibility
Optimized chromatographic conditions ensure the detection of low-abundance impurities while maintaining data consistency and reproducibility, meeting the quality control standards of the biopharmaceutical industry.
4. Customized Services
Tailored chromatographic solutions and analytical methods are provided based on client requirements, addressing the quality control and research needs of various mRNA products, supporting product optimization and process improvement.
Applications
1. mRNA Vaccine Quality Control
mRNA HPLC analysis services are used to assess the purity, integrity, and modifications of mRNA vaccines, ensuring consistency in the production process and compliance with biopharmaceutical quality standards.
2. mRNA-Based Gene Therapy Development
mRNA HPLC enables quality analysis of mRNA gene therapy drugs, including structural modifications, degradation product detection, and stability studies, optimizing manufacturing processes and enhancing therapeutic efficacy.
3. mRNA Modification and Synthesis Optimization
Detecting the impact of different modified nucleotides on mRNA stability and translation efficiency, supporting the improvement of RNA synthesis processes.
4. mRNA Stability and Degradation Studies
mRNA HPLC analysis services assesse mRNA degradation under different pH levels, temperatures, and storage conditions, evaluating long-term stability and providing data support for storage and transportation optimization.
5. mRNA Impurity Analysis
By detecting truncated fragments, double-stranded RNA (dsRNA), and other impurities in mRNA samples, this service ensures product safety and enhances the reliability of downstream applications.
Case Study
1. Anion Exchange HPLC Monitoring of mRNA in Vitro Transcription Reactions to Support mRNA Manufacturing Process Development
This study aims to evaluate the application of anion exchange high-performance liquid chromatography (AEX-HPLC) in monitoring in vitro transcription (IVT) reactions of mRNA to optimize the mRNA manufacturing process and improve product quality. The study subjects are mRNA samples collected at different stages of the IVT process, focusing on the separation and detection of integrity, truncated fragments, template DNA, and dsRNA impurities. The methodology employs AEX-HPLC combined with ultraviolet (UV) and fluorescence (FLD) detection, optimizing mobile phase conditions and gradient elution parameters to enhance mRNA separation efficiency. By comparing different transcription batches, the stability and reproducibility of the method are assessed. The results indicate that AEX-HPLC can precisely distinguish intact mRNA from impurities, demonstrating high sensitivity in detecting dsRNA and incomplete transcription products. Additionally, it enables real-time monitoring of the IVT reaction process, facilitating process optimization. The study concludes that AEX-HPLC is an efficient and reliable mRNA analytical tool for process control and quality assessment of IVT reactions, providing critical support for the production of mRNA vaccines and gene therapies while promoting standardization and optimization of mRNA manufacturing processes.
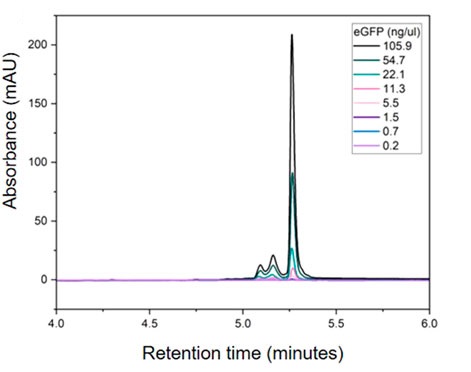
Welbourne, E N. et al. Frontiers in Molecular Biosciences, 2024.
Figure 1. Quantification of mRNA Using AEX HPLC.
Deliverables
1. Comprehensive Experimental Details
2. Materials, Instruments, and Methods
3. Relevant Liquid Chromatography Parameters
4. The Detailed Information of mRNA HPLC Analysis
5. Raw Data
How to order?







