mAb Antibody Sequencing and Recombinant Expression Services
The inauguration of monoclonal antibodies (mAbs), derived from mice, were immunogenic in humans. These molecules were first synthesized in 1975 by Georges Köhler and César Milstein through the use of hybridoma technology. Later, in 1984, another milestone was achieved with the production of chimeric human antibodies via recombinant DNA technology. Hybridoma technology facilitates the creation of mAbs that target specific antigens, and these cell lines are capable of being preserved through long-term cryopreservation. Various specific mAbs have been developed using this technology and these antibodies target specific antigens.
Hybridoma production encompasses five phases: (1) Designing and optimizing the immunogen for the host animal. (2) Immunizing the host with the immunogen to elicit an immune response and encourage B-cell maturation. (3) Harvesting B-cells from the spleen of the host and fusing them with immortalized myeloma cells to produce hybridomas. (4) Conducting several rounds of screening and selection of the resultant hybridomas to identify the best for producing high-quality mAbs. (5) Expanding these specific hybridomas and purifying the antibodies.
1. Antigen Preparation
For a high yield of hybridoma production and gaining good producer of high-affinity IgG antibodies post-cell fusion, effective immunization of animals (typically mice or rats) is essential. The effectiveness of the immunization depends on several factors such as mouse strain, antigen type and strength, and the strategy and schedule of immunization. Immunogens can be whole cells, cellular components, or purified molecules such as proteins, lipids, nucleic acids, among others. The purity of immunogens typically correlates with the specificity of the target antibodies produced by the hybridomas. While whole cells or cell lysates can elicit robust immune responses, they may not specifically target the desired antigens. Conversely, highly purified antigens induce specific immune responses, though the intensity of these responses may vary with the antigen's properties.
2. Animal Immunization
Experimental animals (commonly rabbits or mice) receive a series of antigen-containing injections. This immunization leads to B-cell maturation and differentiation, resulting in the generation of plasma and memory B cells. The animals are euthanized once substantial serum antibody are produced, which indicates sufficient immunization. Injections are typically administered through the footpad every two days on a schedule of five total injections (day 0, 3, 6, 9, and 12). To boost the immune response, additional injections are given one to three times a week (days 15, 22, and 29), with the total number of injections reaching up to eight, which depends on the results of serum titration. Serum samples are collected on day 0 (pre-immunization), day 14 (post-injection), and day 31 (post-immunization schedule) to assess antibody titers and evaluate the immune response throughout and following the immunization process.
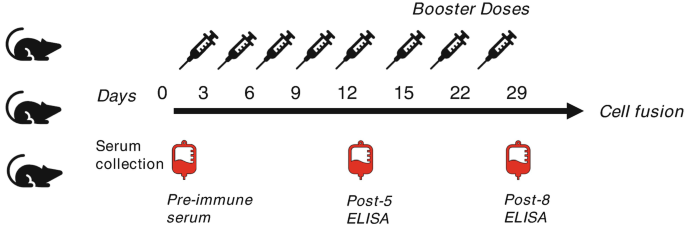
Figure 1. Immunization Schedule [4]
3. Isolation of B Cell and Preparation of Hybridoma Cells
After sacrificing the immunized animals, their spleens are excised under sterile conditions to isolate the activated B cells by density gradient centrifugation. Antibodies in the serum can be detected using methods such as ELISA or flow cytometry. The serum contains activated B lymphocytes that produce antibodies. These activated B lymphocytes are then fused with myeloma cells. The SP2/0 myeloma cell line is selected as the partner cell line for hybridomas. A week prior to fusion, SP2/0 mouse myeloma cells are thawed, cultrured in RPMI-1640 supplemented with 10% FBS, and grown to a density of 1×108 in culture dishes. On the day of fusion, cells are collected in a 50 mL tube through centrifugation, and washed twice with RPMI medium prior to fusion.
Cell fusion involves combining activated B lymphocytes with HAT-sensitive myeloma cells. This process is facilitated by culturing freshly isolated activated B cells with HAT-sensitive myeloma cells in a fusion-promoting culture medium. Polyethylene glycol (PEG) is used to enhance membrane fusion, which can help integrate membranes of the myeloma and antibody-producing cell to form multinucleated cells, known as heterokaryon. Electrofusion is another fusion technique, where cells are merged under an electric field. Short, high-voltage pulses induce a temporary disordered state in the lipids of cell membranes, allowing them to rearrange into a normal bilayer shortly after the pulse. This facilitates fusion when the cell membranes are in close proximity.
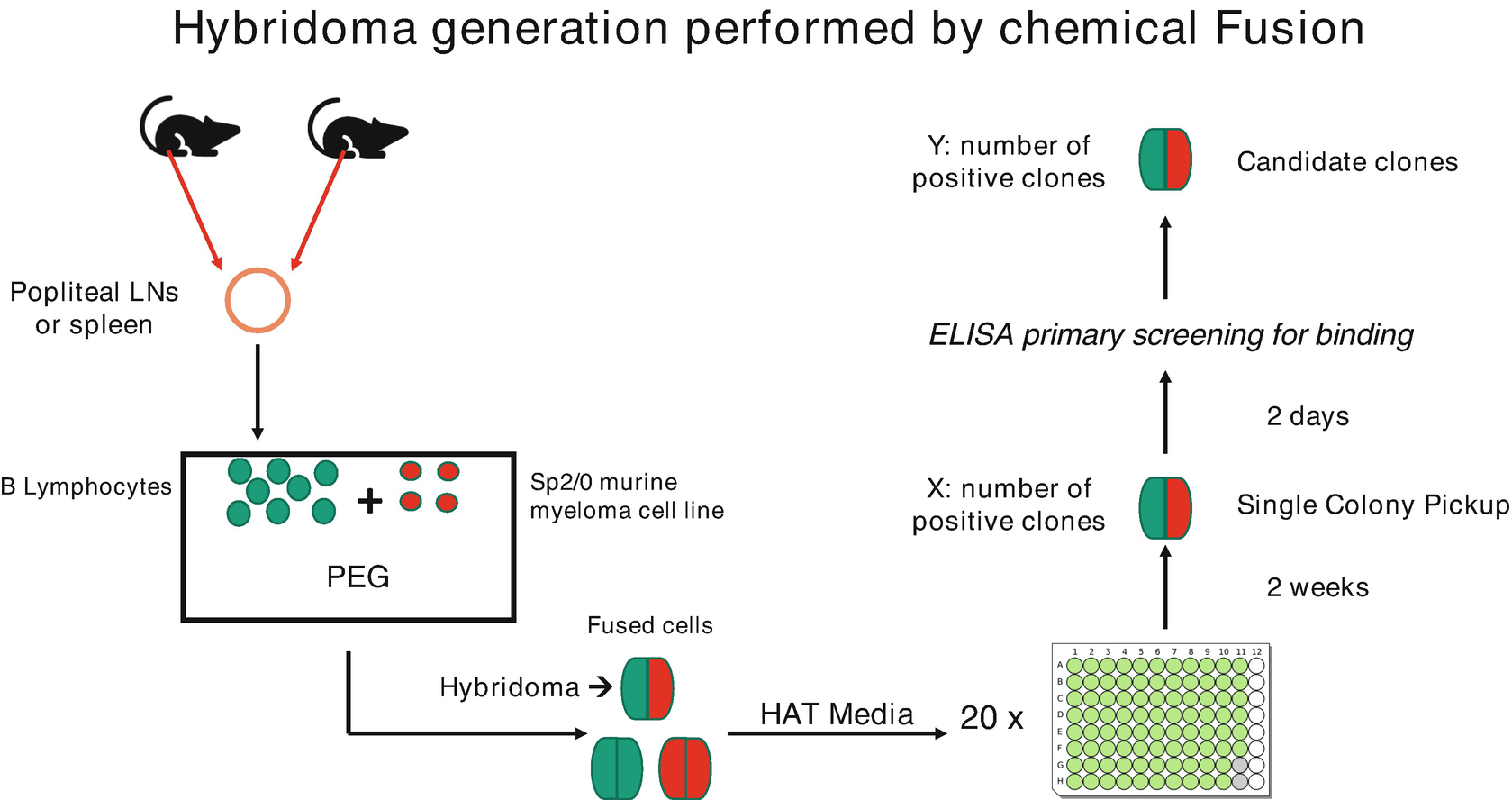
Figure 2. Preparation of Hybridoma Cells [4]
4. Screening of Hybridoma Cells
After selection, hybridoma cells are cultured in HAT medium while unfused myeloma cells and B cells are eliminated. To isolate single clone, the "limited dilution method" is employed post-cell fusion to inoculate cells into microtiter plates. It should ensure approximately one fused cell per well. These hybridoma clones, once established, can be observed under a microscope; their supernatants, containing significant amounts of antibodies, facilitate the screening of productivity and specificity. Established methods for screening include ELISA, surface plasmon resonance (SPR), biolayer interferometry, flow cytometry, and bioassays.
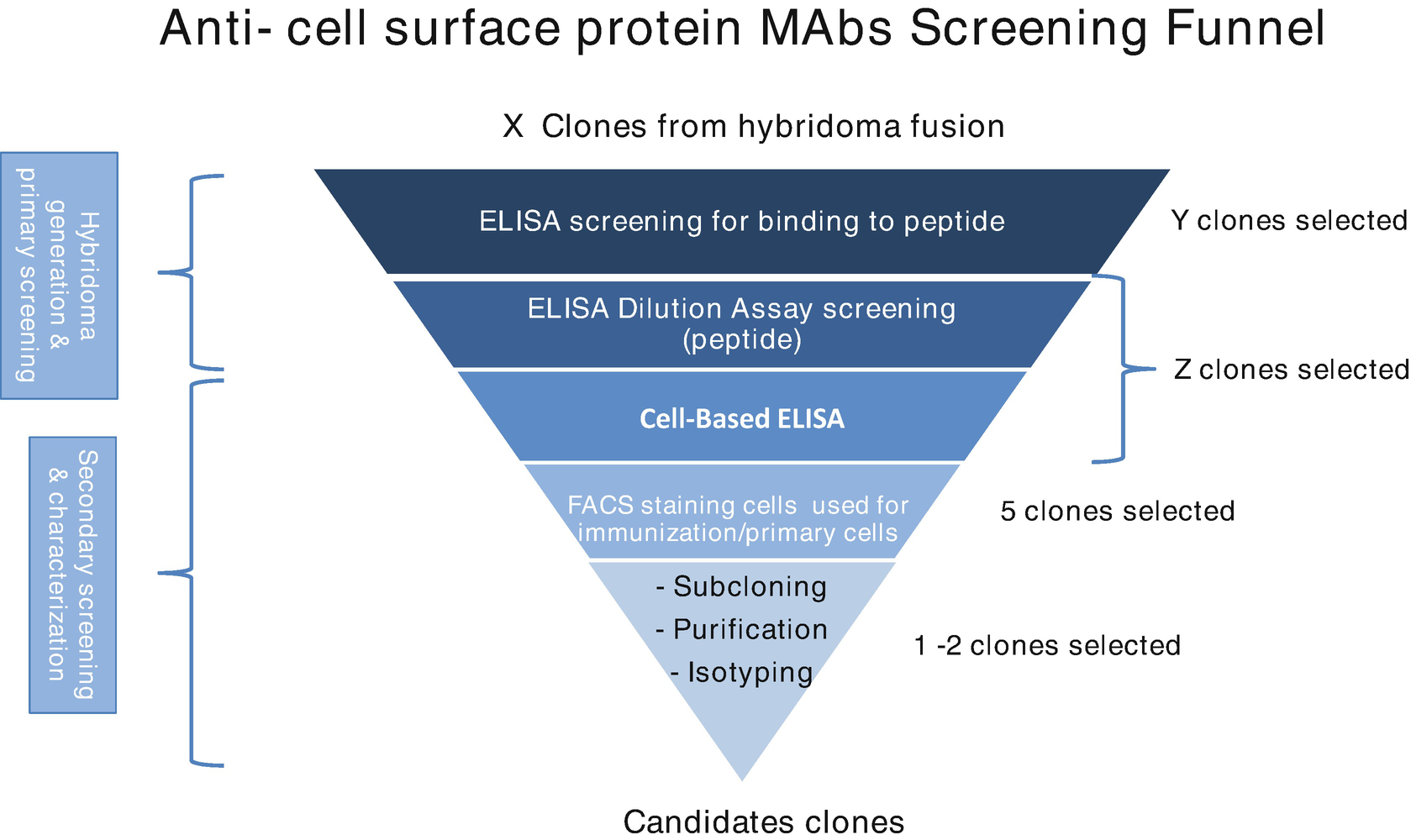
Figure 3. Screening of Hybridoma Cells [4]
5. Expansion and Purification of Specific Hybridomas and Antibodies
Upon confirmation that hybridoma cells are capable of producing the desired antibodies, these cells are then propagated either in vivo using mice as hosts to produce mAbs, or in vitro, cultured in cell culture mediums. For in vivo propagation, hybridoma cells are injected into the peritoneal cavity of mice, and after several weeks, ascites fluid is collected from mice anesthetized. This fluid, contaminated with mouse immunoglobulins, necessitates purification of the mAbs. For in vitro methods, hybridoma cells are obtained in a culture medium.
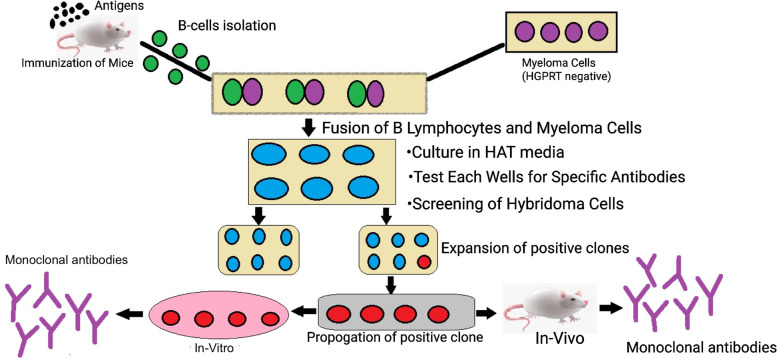
Figure 4. Amplification of Positive Clone Hybridoma Cells [5]
Following amplification and incubation of selected hybridoma cells, the mAb-rich supernatant (whether ascites fluid or cell culture medium) is collected through centrifugation and membrane-filteration. mAbs are then purified using per aqueous liquid chromatography (PALC), with concentrations subsequently measured using a Nanodrop.
6. Hybridoma Cell Antibody Gene Sequencing
Hybridoma cells are at risk of losing high specificity and activity, or may be in suboptimal condition or deceased. Sequencing of hybridoma cells can obtain their sequences, which help stable antibody production via recombinant proteins. The sequencing protocol involves RNA extraction, reverse transcription, PCR amplification of heavy and light chain sequences, sequencing validation, and vector construction.
7. Recombinant Antibody Production
mAb production commences with the selection of an appropriate expression system. Typically mammalian expression system include host cell lines, which synthesizes therapeutic molecules more effective than non-mammalian systems due to their ability to produce proteins with human-like post-translational modifications (PTMs). During the development of mAb-producing cell lines, mammalian cells are transfected with expression constructs containing target genes (target mAb's light and heavy chains). Transfection introduces exogenous genetic material into specific host cells and transfection method can be viral, non-viral, or a hybrid of both methods. Viral methods utilize transduction with a virus (such as herpes virus and adenovirus) as a carrier, while non-viral methods might use chemical or physical/mechanical techniques, such as electroporation based on lipid or non-lipid chemical or physical/mechanical techniques. Hybrid strategies integrate the two methods.
mAb production can be achieved through a stable expression system where the introduced genetic material, including genes for the light and heavy chains and one or more selectable markers, is integrated into the host genome. This integration ensures continuous genetic expression, even post-cell replication. Common host cell lines for recombinant mAb production include CHO, NS0, SP2/0, HEK293, and PER.C6. CHO cells dominate industrial protein therapeutic production and studied in many researches. NS0 and SP2/0 cells can also be used in the production of recombinant mAb and express immunogenic glycan epitopes like alpha-galactose, 3-gal (alpha-galactosidase), and N-glycolylneuraminic acid (Neu5Gc). HEK293 and PER.C6 cells are noted for their glycosylation patterns matching that of human tissues. Nonetheless, CHO-derived mAbs, exhibiting higher sialic acid levels than those from HEK cells, which is of concern to in vivo heterogeneity, particularly in mAb production. HEK293 cells are ideal for transient gene expression, allowing for protein harvest within several days of DNA transfection.
Analysis Workflow
1. Establishment of Experimental Protocols According to Study Requirements
2. Antigen Preparation and Animal Immunization
3. Preparation of Hybridoma Cells
4. Screening of Hybridoma Cells
5. Sequencing of Hybridoma Cell Antibody Genes
6. Expression of Recombinant Antibodies
Service Advantages
1. Preparation of Monoclonal Antibodies Across Multiple Species
2. Comprehensive Preparation and Screening Services for Hybridoma Cells
3. High-Accuracy Antibody Gene Sequencing
4. Effective Construction and Expression of Antibody Gene Expression Vectors
Example Results
1. Development and Application of a Novel Anti-FXYD5 Monoclonal Antibody for Pancreatic and Lung Cancer
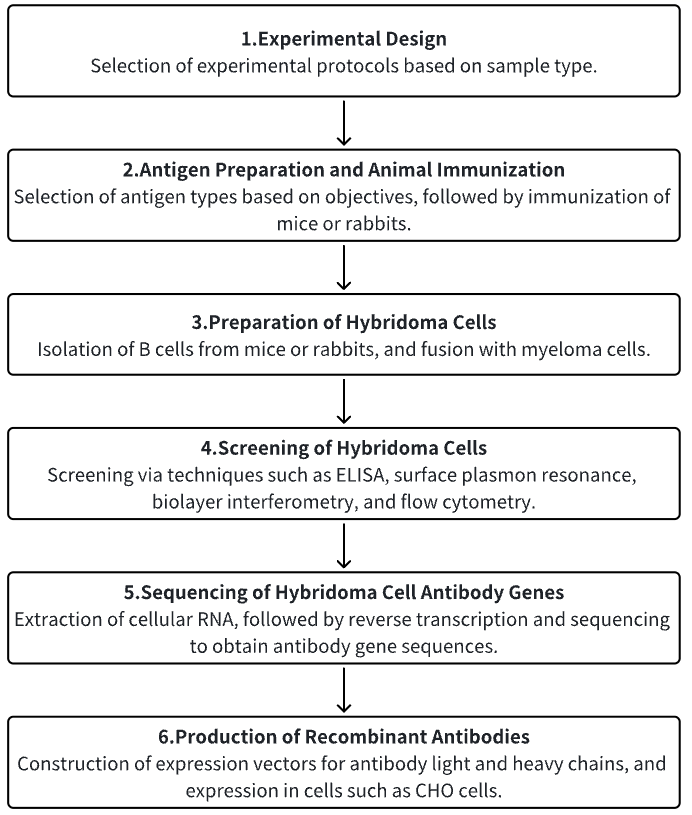
Despite advancements in cancer therapies, pancreatic cancer continues to demonstrate a dire prognosis worldwide. Targeting cancer-specific antigens, exclusively expressed on tumor cells, may mitigate these challenges. Such antigens are potential targets for antibodies in both fundamental research and clinical practice. In efforts to produce an antibody specific to surface proteins of pancreatic cancer cells, researchers immunized mice with human pancreatic cancer MIA PaCa-2 cells and isolated a hybridoma producing a mAb, designated as 12-13.8. This antibody has been utilized in various molecular biology techniques including immunocytochemistry (ICH), immunoblotting (IB), flow cytometry (FCM), and immunoprecipitation (IP). Moreover, it has been demonstrated that mAb 12-13.8 accumulated within tumors in cancer-bearing mice used in in vivo studies. Immunohistochemical staining of pancreatic and lung tumor tissues revealed that increased staining intensity of mAb 12-13.8 was positively correlated with patient's cancer recurrence rate and inversely correlated with survival rate. Through a human protein array screening system, the FXYD5 protein was identified as the target of mAb 12-13.8. Overexpressed in various cancers and modified by O-linked glycosylation, FXYD5's binding to mAb 12-13.8 was confirmed using FXYD5-knockout MIA PaCa-2 cells and and detailed epitope mapping identifified amino acid residues 45-52 as the minimal peptide sequence. These findings indicated that mAb 12-13.8 was a valuable tool for FXYD5 research, with significant implications for the diagnosis and drug delivery applications in oncology.
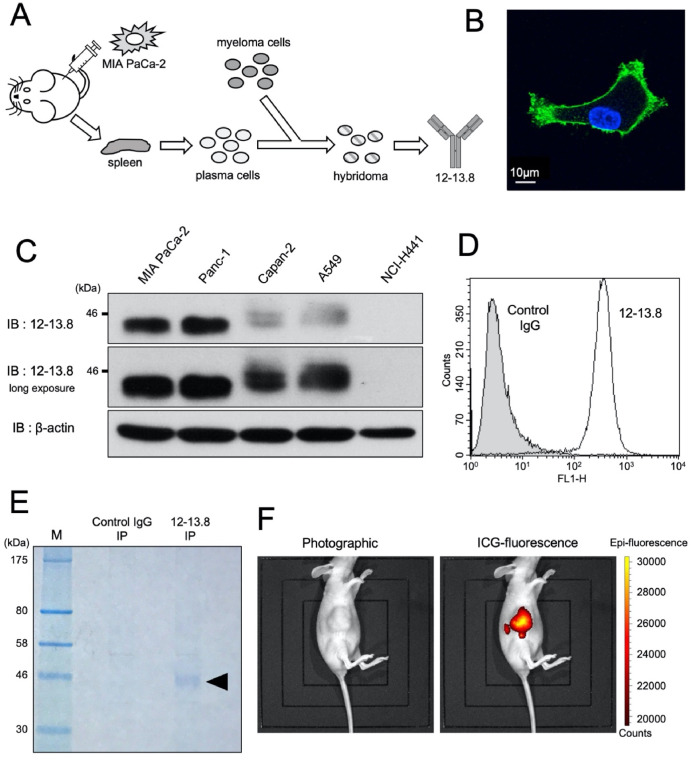
Figure 5. Generation and Application of mAb 12-13.8 [6]
2. De Novo Sequencing and Development of a Unique Antibody for Detecting Alternative Conformations of Cytochrome c in Cells
Cytochrome c (cyt c) is a prevalent heme protein that, in its native conformation, plays essential roles in mitochondrial bioenergetics and apoptotic signaling in mammalian cells. Its dynamic properties provides significant plasticity, allowing for the in vitro generation of alternative conformations, although their detection in fibrous tissues has not been thoroughly investigated. A unique mAb (R1D3) that can identify conformational changes in cyt c proteoforms was developed using MS-based de novo sequencing. The deployment of R1D3 across different cell models showed that these alternative conformations of cyt c could move from the mitochondria to the nuclei without inducing apoptosis. R1D3 served as a distinctive immunochemical tool in mitochondrial and cellular biology, facilitating insights into protein dynamics and plasticity, and identifying the noncanonical structures and functions of cyt c in vivo.
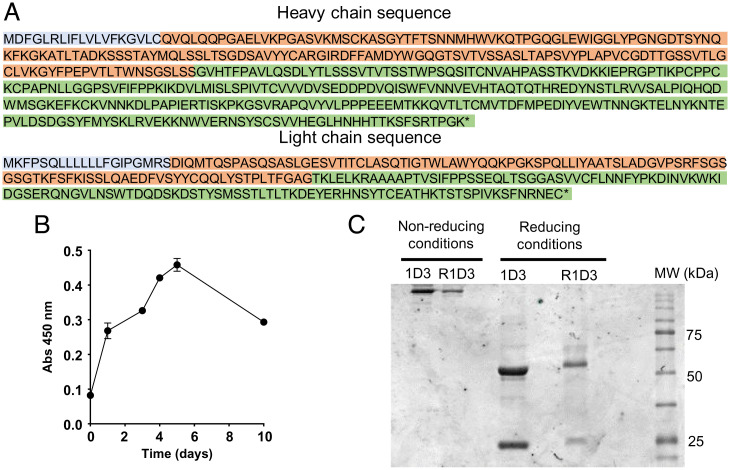
Figure 6. Production of the R1D3 Antibody Based on mAb 1D3 [7]
3. A Novel Rabbit Anti-Myoglobin Monoclonal Antibody's for Potential Use in Acute Kidney Injury Associated with Rhabdomyolysis
Myoglobin (Mb), a primary component of vertebrate skeletal and muscle and myocardium, is crucial for oxygen binding, storage, and transport, and plays a vital role in earliest disease diagnostics. Novel recombinant rabbit anti-Mb mAbs were developed for diagnosing Mb deposition in rhabdomyolysis-associated acute kidney injury (RM-AKI). The cloning and expression of the full-length coding sequence of rat Mb led to the creation of high-quality, titer rabbit anti-Mb polyclonal antibodies using the immunogen His-Mb fusion protein. New hybridoma cells were derived through hybridoma screening methods, and expression plasmids for the anti-Mb monoclonal antibodies' heavy and light chains were constructed via DNA sequencing and molecular clonal. These antibodies demonstrated exceptionally high affinity (KD= 1.21 pM), broad species reactivity (including mouse, rat, human, and horse), and specific affinity for skeletal muscle and myocardium. They also showed excellent performance in detecting Mb levels in RM-AKI affected kidneys, myocardium, and skeletal muscles through Western blot (WB), ICH, and immunofluorescence (IF).
Figure 7. Preparation of the Immunogen His-Mb Protein and the Generation of Plasma-Derived Rabbit Anti-Mb Polyclonal Antibodies [8]
Sample Submission Requirements
1. Minimization of Impurity Contamination
Services at MtoZ Biolabs
1. Comprehensive Experimental Procedures
2. Detailed Specifications of Instrumental Parameters
3. Original Experimental Data
4. Data Analysis Report
Applications
1. Database Development
The NeuroMabSeq project, a joint initiative, focuses on identifying and disclosing mAb hybridoma-derived sequences for neuroscience research. Spanning over three decades of research and development, primarily at the University of California, Davis/NIH NeuroMab Facility, this effort has yielded numerous neuroscience-validated mouse mAbs. To improve dissemination and enhance the utility of this critical resource, studies employing high-throughput DNA sequencing have mapped immunoglobulin heavy and light chain variable domains from the original hybridoma cells. The sequences are now accessible through a searchable DNA sequence database at neuromabseq.ucdavis.edu, facilitating sharing, analysis, and use in downstream applications.
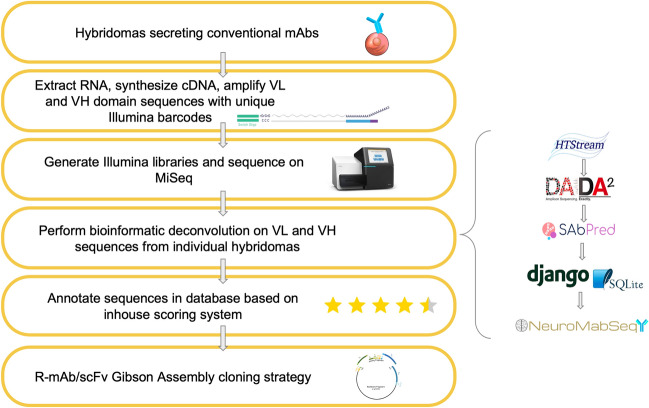
Figure 8. Development of the NeuroMab Database[9]
2. Applications of Recombinant Antibodies in Therapy, Detection, and Other Areas
Antibodies are fundamental tools in both biological and biochemical research and are increasingly utilized in novel therapeutic areas. Serving as crucial probes, they help elucidate protein functions in both normal and pathophysiological conditions. Moreover, antibodies are indispensable for quantifying biomarkers, monitoring temporal and spatial patterns of protein expression and identifying interactive partners along with their biological activities.
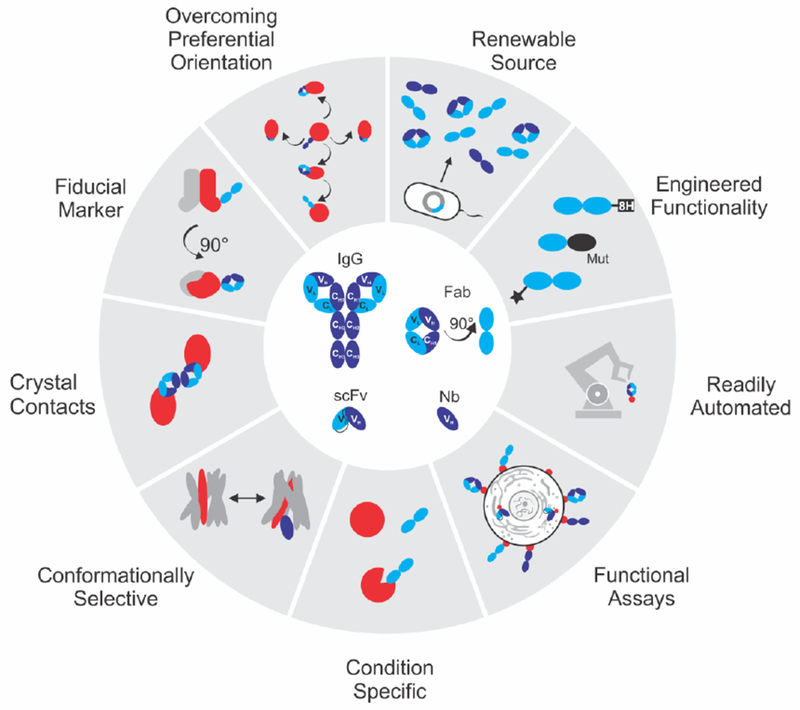
Figure 9. Applications of Recombinant Antibodies[10]
FAQ
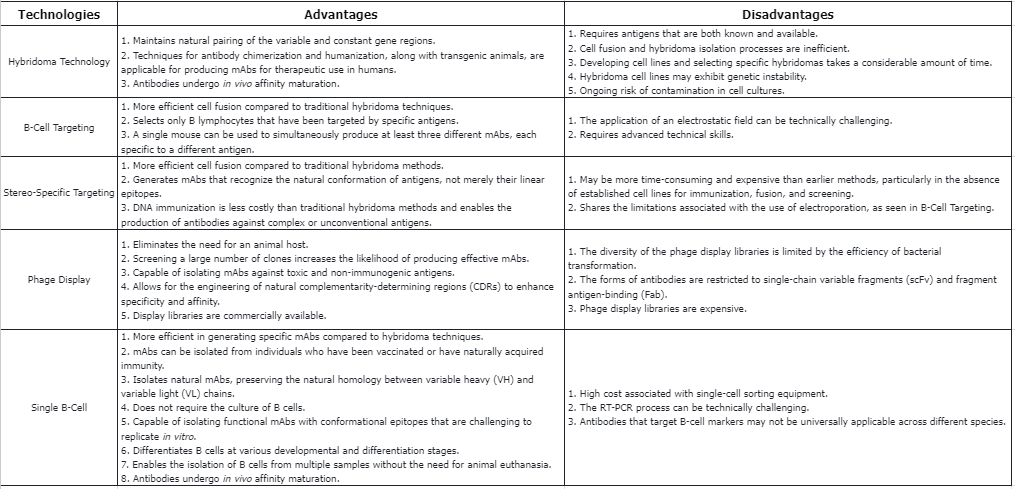
Q1: What are the advantages and disadvantages of technologies used to generate mAbs?
References
[1] Snapkov I, Chernigovskaya M, Sinitcyn P, Lê Quý K, Nyman TA, Greiff V. Progress and challenges in mass spectrometry-based analysis of antibody repertoires. Trends Biotechnol. 2022 Apr;40(4):463-481. doi: 10.1016/j.tibtech.2021.08.006. Epub 2021 Sep 14. PMID: 34535228.
[2] de Brito PM, Saruga A, Cardoso M, Goncalves J. Methods and cell-based strategies to produce antibody libraries: current state. Appl Microbiol Biotechnol. 2021 Oct;105(19):7215-7224. doi: 10.1007/s00253-021-11570-x. Epub 2021 Sep 15. PMID: 34524471.
[3] Alejandra WP, Miriam Irene JP, Fabio Antonio GS, Patricia RR, Elizabeth TA, Aleman-Aguilar JP, Rebeca GV. Production of monoclonal antibodies for therapeutic purposes: A review. Int Immunopharmacol. 2023 Jul;120:110376. doi: 10.1016/j.intimp.2023.110376. Epub 2023 May 25. PMID: 37244118.
[4] Muhsin, A., Rangel, R., Vien, L., Bover, L. (2022). Monoclonal Antibodies Generation: Updates and Protocols on Hybridoma Technology. In: McAllister, F. (eds) Cancer Immunoprevention. Methods in Molecular Biology, vol 2435. Humana, New York, NY. https://doi.org/10.1007/978-1-0716-2014-4_6.
[5] Mitra S, Tomar PC. Hybridoma technology; advancements, clinical significance, and future aspects. J Genet Eng Biotechnol. 2021 Oct 18;19(1):159. doi: 10.1186/s43141-021-00264-6. PMID: 34661773; PMCID: PMC8521504.
[6] Hotta T, Nariai Y, Kajitani N, Kadota K, Maruyama R, Tajima Y, Isobe T, Kamino H, Urano T. Generation of the novel anti-FXYD5 monoclonal antibody and its application to the diagnosis of pancreatic and lung cancer. Biochimie. 2023 May;208:160-169. doi: 10.1016/j.biochi.2023.01.002. Epub 2023 Jan 5. PMID: 36621663.
[7] Tomasina F, Martínez J, Zeida A, Chiribao ML, Demicheli V, Correa A, Quijano C, Castro L, Carnahan RH, Vinson P, Goff M, Cooper T, McDonald WH, Castellana N, Hannibal L, Morse PT, Wan J, Hüttemann M, Jemmerson R, Piacenza L, Radi R. De novo sequencing and construction of a unique antibody for the recognition of alternative conformations of cytochrome c in cells. Proc Natl Acad Sci U S A. 2022 Nov 22;119(47):e2213432119. doi: 10.1073/pnas.2213432119. Epub 2022 Nov 15. PMID: 36378644; PMCID: PMC9704708.
[8] Wang X, Qiao O, Han L, Li N, Gong Y. A Novel Rabbit Anti-Myoglobin Monoclonal Antibody's Potential Application in Rhabdomyolysis Associated Acute Kidney Injury. Int J Mol Sci. 2023 Apr 25;24(9):7822. doi: 10.3390/ijms24097822. PMID: 37175528; PMCID: PMC10177957.
[9] Mitchell KG, Gong B, Hunter SS, Burkart-Waco D, Gavira-O'Neill CE, Templeton KM, Goethel ME, Bzymek M, MacNiven LM, Murray KD, Settles ML, Froenicke L, Trimmer JS. High-volume hybridoma sequencing on the NeuroMabSeq platform enables efficient generation of recombinant monoclonal antibodies and scFvs for neuroscience research. Sci Rep. 2023 Sep 27;13(1):16200. doi: 10.1038/s41598-023-43233-4. PMID: 37758930; PMCID: PMC10533561.
[10] Basu K, Green EM, Cheng Y, Craik CS. Why recombinant antibodies - benefits and applications. Curr Opin Biotechnol. 2019 Dec;60:153-158. doi: 10.1016/j.copbio.2019.01.012. Epub 2019 Mar 5. PMID: 30849700; PMCID: PMC6728236.
How to order?







