In-Situ X-ray Photoelectron Spectroscopy (XPS) Analytical Service
In-situ X-ray photoelectron spectroscopy (XPS) analytical is a high-precision surface analysis technique that enables real-time monitoring of changes in elemental composition and chemical states on material surfaces under controlled atmospheres, temperatures, voltages, or reaction environments. Its basic principle involves irradiating the sample surface with X-rays to eject core-level electrons, then measuring their kinetic energy to calculate binding energies, thereby identifying elemental types, valence states, and chemical environment changes. Unlike conventional XPS, in-situ XPS allows dynamic monitoring of material interfaces during catalytic reactions, electrochemical processes, thermal treatments, or plasma exposure without disturbing the reaction conditions. It features strong temporal resolution, high spatial resolution, and atomic-level sensitivity.
In-situ X-ray photoelectron spectroscopy (XPS) analytical service is widely applied in cutting-edge research areas such as electrocatalysis (e.g., OER, HER, CO₂RR), battery electrode/electrolyte interface studies, monitoring of metal corrosion behavior, thin film growth processes, and investigations of new material synthesis and aging behavior. It is an indispensable analytical tool in energy materials and surface science.

Ohtsu, N. et al. Langmuir, 2009.
Figure 1. In-situ XPS Measurement System.
Services at MtoZ Biolabs
Based on a high-resolution in-situ XPS analytical platform equipped with an atmosphere-controlled reaction chamber and heating/biasing capabilities, MtoZ Biolabs’ in-situ X-ray photoelectron spectroscopy (XPS) analytical service enables real-time monitoring of changes in surface elemental composition, chemical valence states, and electronic structure under realistic working conditions. This service provides atomic-level surface elemental distribution data and dynamic evolution information in response to variations in reaction time, temperature, or atmosphere. It is ideally suited for studies in catalysis, electrochemistry, and interfacial material behavior, ensuring precise, reliable, and highly reproducible results.
Analysis Workflow
1. Sample Preparation and Loading
The sample should be prepared in a size and form suitable for XPS analysis, with a clean and flat surface, and then loaded into the in-situ XPS vacuum chamber.
2. In-situ Condition Setup
Based on the research objectives, appropriate in-situ experimental conditions are set, including temperature (via controlled heating), atmosphere (e.g., reactive or inert gases such as H₂, O₂, CO₂), and electrical bias, to simulate real working environments.
3. X-ray Irradiation and Photoelectron Collection
Under the defined conditions, a monochromatic X-ray source irradiates the sample surface to excite photoelectrons. An electron energy analyzer is used to collect and analyze the energy spectra corresponding to different elements and their chemical states.
4. Real-time Monitoring and Data Acquisition
During the reaction, photoelectron spectra are recorded in real-time to capture changes in elemental valence states and binding energies, enabling the tracking of intermediate species and interfacial evolution.
5. Data Analysis and Reporting
Collected spectra are subjected to peak fitting and both qualitative and quantitative analysis. A comprehensive report is generated, detailing elemental composition, changes in chemical states, and insights into reaction mechanisms.
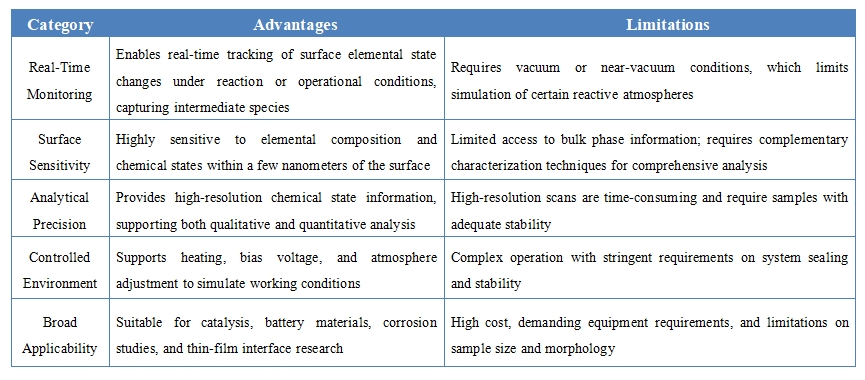
Advantages and Limitations
Applications
1. Catalytic Reaction Mechanism Studies
In-situ X-ray photoelectron spectroscopy (XPS) analytical service enables real-time monitoring of changes in elemental valence states and the formation and transformation of reaction intermediates on catalyst surfaces under reaction conditions, revealing reaction pathways and the dynamic evolution of active sites.
2. Failure Analysis and Stability Validation
By tracking changes in surface elemental composition and chemical states under complex service environments, this technique helps uncover degradation mechanisms and guides the optimization of material design.
3. Interfacial Behavior of Energy Materials
In-situ X-ray photoelectron spectroscopy (XPS) analytical service is suitable for studying interfacial reconstruction, charge accumulation, and film formation processes in systems such as fuel cells, water electrolysis, and battery electrode materials during electrochemical reactions.
4. Surface Modification and Coating Evaluation
This service monitors changes in surface composition and chemical states during heating, plasma treatment, or gas exposure to evaluate the effectiveness of surface modifications and the stability of functional coatings.
5. Atmospheric Response Studies
In-situ X-ray photoelectron spectroscopy (XPS) analytical service enables direct observation of surface responses under high-temperature, reducing, or oxidizing atmospheres, supporting the study of adsorption, desorption, and interfacial reactions.
Case Study
1. Initial Oxidation of U₃Si₂Studied by In-situ XPS Analysis
This study aims to investigate the chemical state evolution during the initial oxidation process of U₃Si₂ in an oxygen environment using in-situ X-ray photoelectron spectroscopy (XPS). The research focuses on the oxidation behavior of the U₃Si₂ surface at different temperatures. Through in-situ XPS analysis, the valence changes of U and Si elements and the evolution of surface oxygen content were systematically monitored. Combined with heating treatment and stepwise oxygen exposure experiments, the study elucidated the time–composition relationship of the oxidation process. Results show that surface oxidation of U₃Si₂ occurs even at low temperatures, leading to the formation of UO₂, along with the gradual generation of SiO₂. At elevated temperatures, the proportion of higher-valence uranium oxides increases. The study concludes that the oxidation behavior of U₃Si₂ exhibits multi-stage characteristics, and in-situ XPS provides time-resolved electronic structure information critical for understanding the surface oxidation mechanism, thereby supporting the evaluation of its stability and oxidation resistance in nuclear fuel applications.
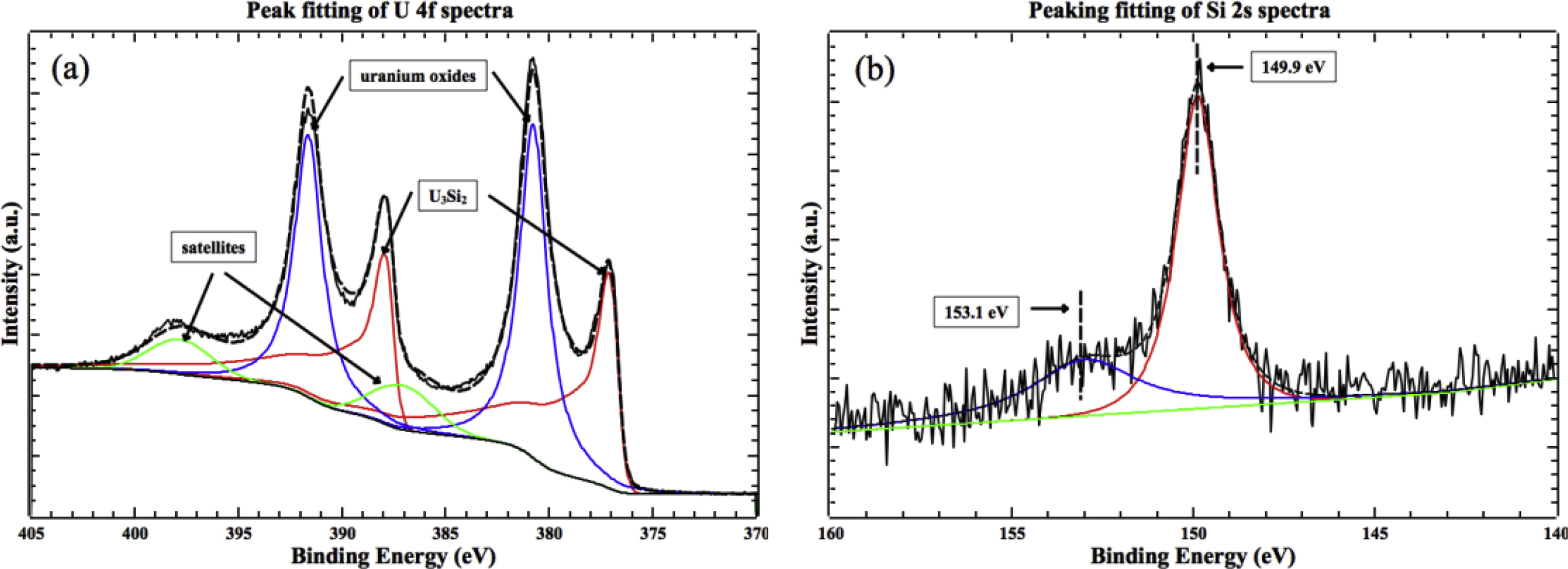
Yan, T W. et al. Journal of Nuclear Materials, 2019.
Figure 2. Examples of Peak Fitting Process.
2. In-situ XPS Analysis of Oxidized and Reduced Plasma Deposited Ruthenium-based Thin Catalytic Films
This study aims to investigate the surface chemical changes of plasma-deposited ruthenium-based catalytic thin films under oxidation and reduction conditions using in-situ X-ray photoelectron spectroscopy (XPS). The research focuses on Ru thin film catalysts modulated by different treatment atmospheres (oxygen and hydrogen). By monitoring the valence state of Ru and its bonding with oxygen or hydrogen through in-situ XPS, the study reveals that Ru rapidly oxidizes to form RuO₂ in an oxygen atmosphere, while under hydrogen reduction conditions, Ru oxides are gradually reduced to metallic Ru. The results demonstrate that the chemical state of Ru in the film can be reversibly tuned by controlling the gas atmosphere, and the reaction process can be tracked in real time via in-situ XPS. The study concludes that in-situ XPS is an effective tool for dynamically analyzing the electronic structure evolution of catalytic materials under reaction conditions and provides important insights for mechanistic studies and performance optimization of ruthenium-based catalysts.
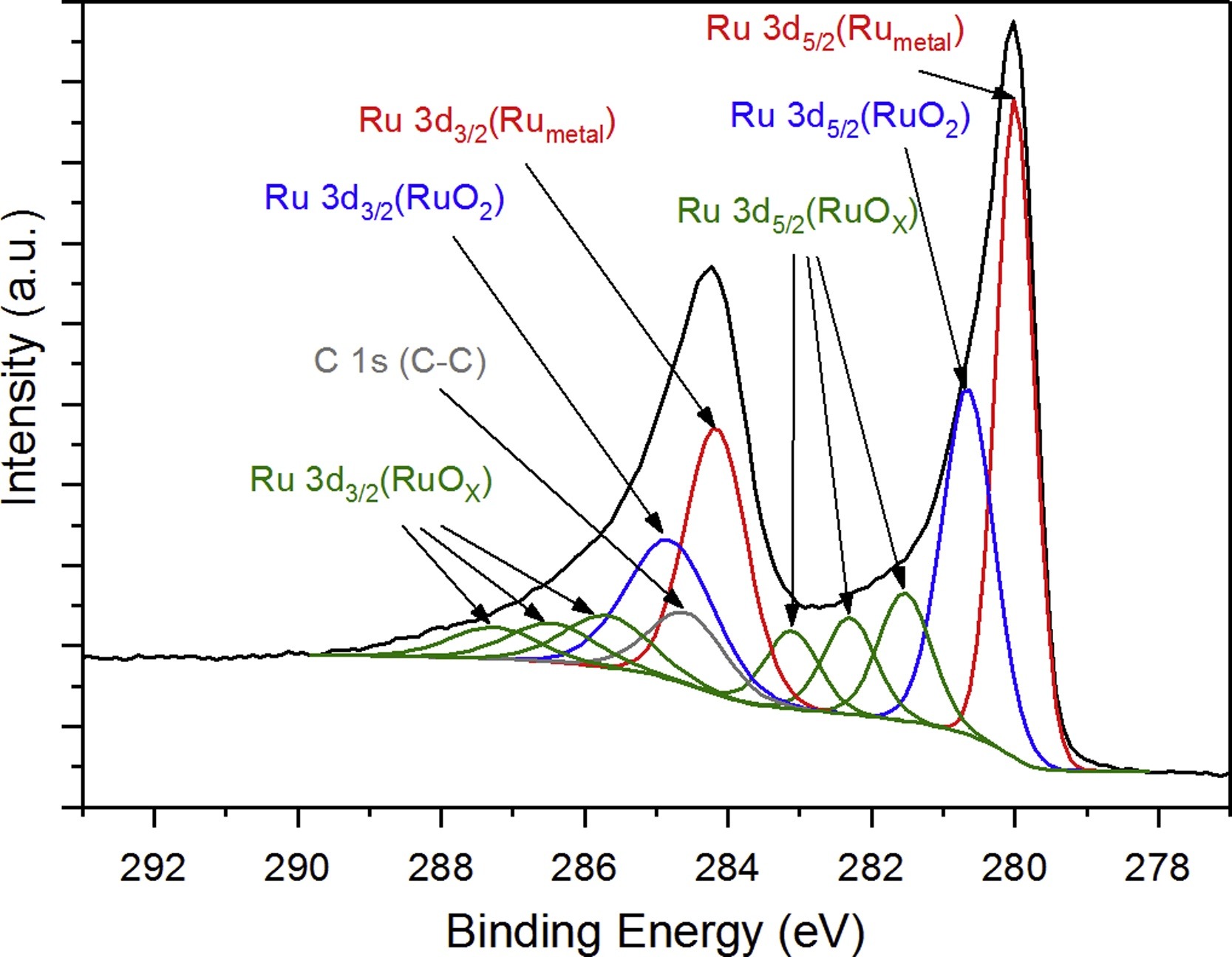
Balcerzak, J. et al. Applied Surface Science, 2017.
Figure 3. XPS Ru 3d Resolved Spectrum of As-deposited Ru-based Film.
FAQ
Q1: What Is the Difference between In-situ XPS and Conventional XPS?
A1: Conventional XPS can only analyze static samples under ultra-high vacuum conditions, whereas in-situ XPS enables real-time analysis of samples under practical working atmospheres (such as H₂, O₂, CO, CO₂) and temperature conditions. It allows dynamic tracking of changes in surface elemental valence states and chemical environments, making it suitable for studying reaction mechanisms and interfacial behaviors.
Q2: What Types of Materials Are Suitable for In-situ XPS?
A2: In-situ XPS is applicable to metals, alloys, oxides, sulfides, nitrides, catalysts, energy storage materials, and coating materials. It is particularly suitable for surface-sensitive materials and those with strong environmental responsiveness.
How to order?







