High Throughput Screening Significance in Drug Discovery
Introduction: Core Technologies Transforming the Drug Development Paradigm
The high throughput screening significance in drug discovery has become increasingly prominent as it redefines the traditional landscape of pharmaceutical research and development. Serving as a core engine of modern drug discovery, high throughput screening (HTS) enables the rapid assessment of biological activity across vast compound libraries by integrating automated technologies. This transformative approach significantly shortens the time required for hit identification—from several months using conventional strategies to just a few weeks—while enhancing experimental throughput to accommodate millions of samples in a single run.
The high throughput screening significance in drug discovery is further reflected in its widespread industrial adoption. In fact, more than 60% of novel drug development projects from the top 20 global pharmaceutical companies rely heavily on HTS, making it a critical and irreplaceable platform for early-phase compound identification. Mechanistically, HTS leverages robotics, microfluidic systems, and sensitive detection instruments to achieve advances in three key areas: (1) efficiency—enabling a single day of HTS to match months of manual experiments; (2) cost-effectiveness—reducing per-sample testing costs by two orders of magnitude; and (3) scientific value—producing high-dimensional data sets essential for training AI-driven drug design (AIDD) algorithms. Typical applications include screening for GPCR agonists, activity profiling of kinase inhibitor libraries, and rapid discovery of inhibitors targeting the SARS-CoV-2 main protease.
HTS Methodologies and Implementation Strategies
1. Standardized Workflow
(1) Target Validation: CRISPR gene editing is employed to construct reporter cell lines, ensuring the biological relevance of screening targets.
(2) Compound Library Management: A two-dimensional barcode system manages libraries containing between 500,000 and 2 million small molecules.
(3) Automated Dispensing: Nanoliter-precision dispensing technology maintains pipetting error rates below 5%.
(4) Multimodal Detection: Up to six detection modes—such as fluorescence polarization (FP) and time-resolved fluorescence resonance energy transfer (TR-FRET)—are integrated to ensure versatile assay compatibility.
The high throughput screening significance in drug discovery also manifests through the standardization of protocols that reduce variability and improve reproducibility across large-scale experiments.
2. Critical Quality Control Metrics
(1) Z’-Factor Monitoring: Each screening batch must maintain a Z’ > 0.5 to guarantee a reliable signal window.
(2) Dynamic Range Calibration: An 8-point standard curve ensures linear response with R² > 0.98.
(3) Inter-Plate Variability: ANOVA is used to control the coefficient of variation (CV) to under 15%.
3. Comparative Advantage Matrix
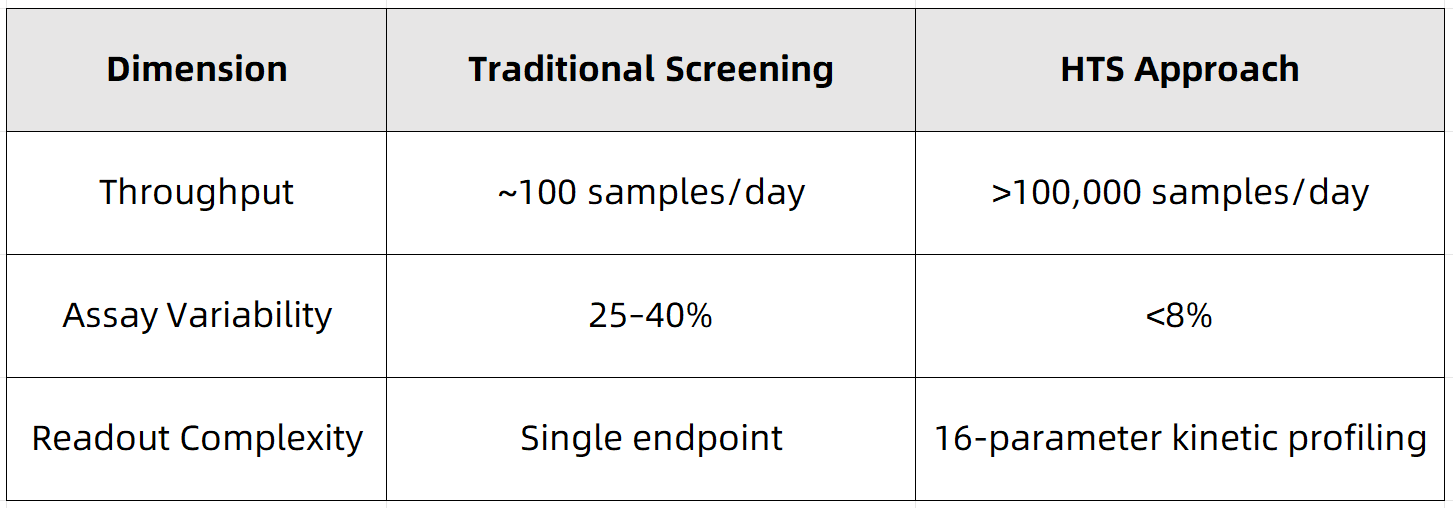
Figure 1
Implementation Challenges and Mitigation Strategies
1. Sample Evaporation
Edge-well evaporation in 384-well plates can distort signal intensity by as much as 30%. To counter this, the integration of humidity-controlled modules with low-evaporation sealing membranes has effectively reduced the coefficient of variation to below 7%.
2. Signal Interference
Intrinsic compound fluorescence often causes false positives. The use of dual-wavelength ratiometric analysis combined with delayed-readout strategies reduces interference rates from 15% to 2.3%, thereby improving screening fidelity. The high throughput screening significance in drug discovery becomes particularly evident in these contexts, where technical challenges are mitigated by tailored engineering solutions that preserve data integrity and operational scalability.
3. Data Integration
High-throughput campaigns generate terabyte-scale data sets requiring robust computational frameworks. Cloud-based distributed computing enables real-time analysis of over 2 million data points per hour—an 80-fold increase in speed compared to traditional data processing systems.
Enabling Drug Discovery with Innovative HTS Services
MtoZ Biolabs has established a next-generation, intelligent HTS platform that has facilitated the successful identification of lead compounds for several innovative pharmaceutical companies. Key strengths of our service platform include:
1. Customized target validation protocols that ensure biologically meaningful screening outcomes.
2. A proprietary compound activity clustering algorithm that identifies potential hits overlooked by conventional scoring.
3. Full-process services encompassing everything from primary screening to lead optimization.
For researchers seeking to unlock the high throughput screening significance in drug discovery, our scientific advisory team is available to develop tailored solutions that accelerate innovation while maintaining scientific rigor.
The Strategic Role of HTS in the Drug Discovery Pipeline
HTS plays a foundational role across critical phases of drug development:
1. Hit Identification
Enables efficient identification of initial hit compounds with desirable biological activity from libraries containing tens of thousands of candidates.
2. Lead Optimization
Structural modifications based on structure–activity relationships (SAR) enhance efficacy, selectivity, and pharmacokinetics.
3. Mechanism-of-Action and Target Validation
HTS integrates with high-content screening (HCS), transcriptomics, and proteomics to investigate compound mechanisms and potential off-target pathways.
The high throughput screening significance in drug discovery is underscored by its ability to detect viable candidates among hundreds of thousands of compounds—an achievement once deemed nearly impossible. With the continued convergence of automation, multi-omics technologies, and AI-based analytics, HTS is evolving from a simple screening tool into a comprehensive platform for systematic mechanism-based studies.
MtoZ Biolabs supports academic and industrial partners worldwide by offering a one-stop solution that integrates high throughput screening, mechanistic investigation, and validation studies. Powered by diverse biological models and advanced proteomics platforms, our services aim to maximize the scientific value of every hit compound and facilitate the transition from hit to lead to candidate drug.
MtoZ Biolabs, an integrated chromatography and mass spectrometry (MS) services provider.
Related Services
How to order?







