HCP Assay Kit Development Service
Commercial ELISA kits play a crucial role in the preliminary phases of drug development. These kits are applicable to drugs already on the market, provided that their detection methodologies have undergone specific process performance validation. In instances where available commercial ELISA kits do not offer adequate coverage of HCPs, the development of product-specific ELISA kits or the employment of platform ELISA kits is advisable. In the process of producing HCP antibodies, the selection of the antigen for immunization and the characterization of its properties, as well as consideration of the species for HCP antigen immunization, are crucial. Regardless of the type of HCP-ELISA utilized—be it commercial, platform, or product-specific—conducting validation tests to ensure suitability for the specific process and product is imperative. This includes choosing HCP standards that accurately represent the sample, verifying quantification dilution linearity, assessing the sensitivity and limit of quantification (LOQ) for the drug samples, and elucidating the characteristics of critical reagents, namely HCP antigens and HCP-specific antibodies.
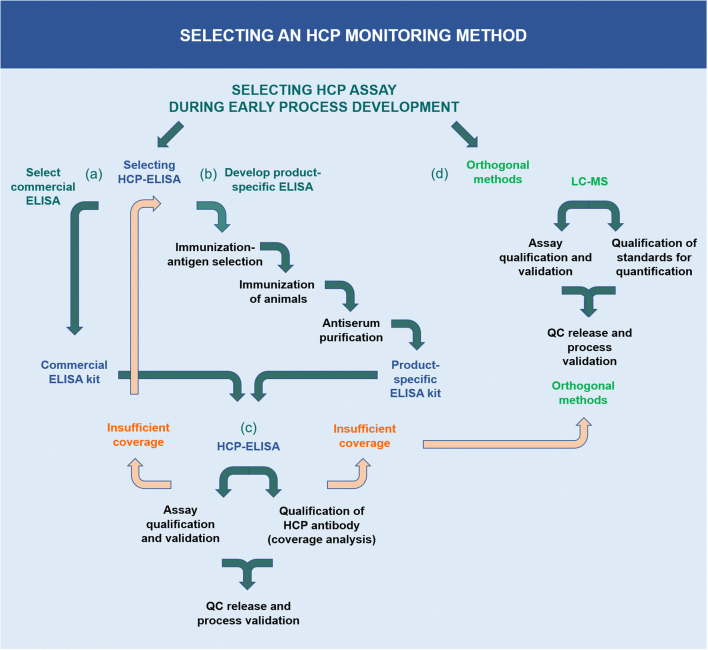
Figure 1. HCP Detection Method [3]
ELISA serves as a pivotal immunological technique for the detection of target substances. This method involves the immobilization of antigens or antibodies onto a solid-phase carrier and leverages the specific interaction between antigens and antibodies, coupled with enzymatic catalysis on marked antibodies or antigens, to induce a colorimetric reaction. This enables detection at picomolar (pmol) concentrations. Typically, ELISA employs dual antibodies: one to capture the antigen on the microtiter plate and another, enzyme-linked antibody for detection and signal amplification. ELISA can be conducted through various methodologies, including homogeneous, heterogeneous, direct, indirect, sandwich, and competitive assays.
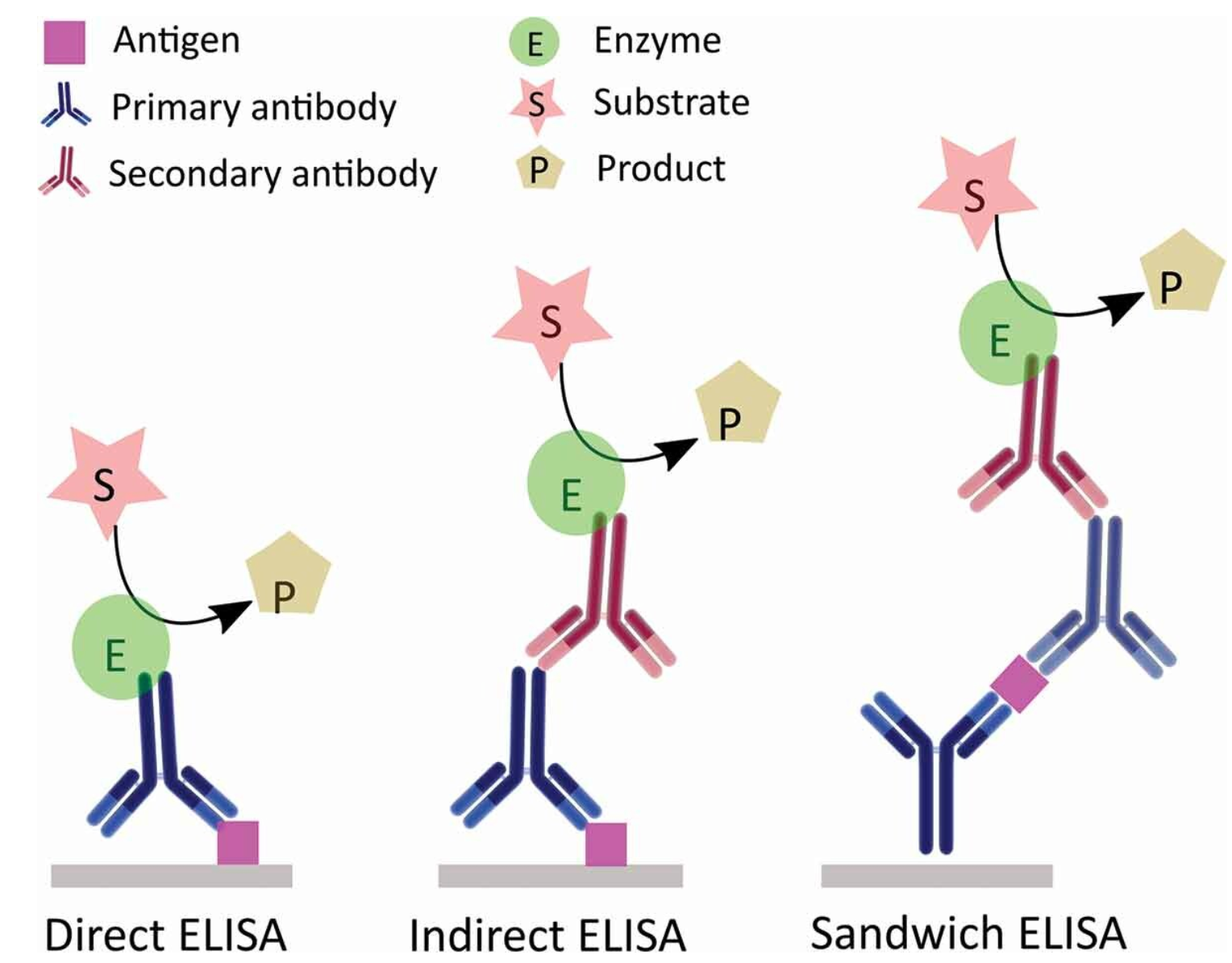
Figure 2. Schematic Diagram of Various ELISA Principles [4]
ELISA Kit Preparation Process
1. Antigen or Antibody Labeling
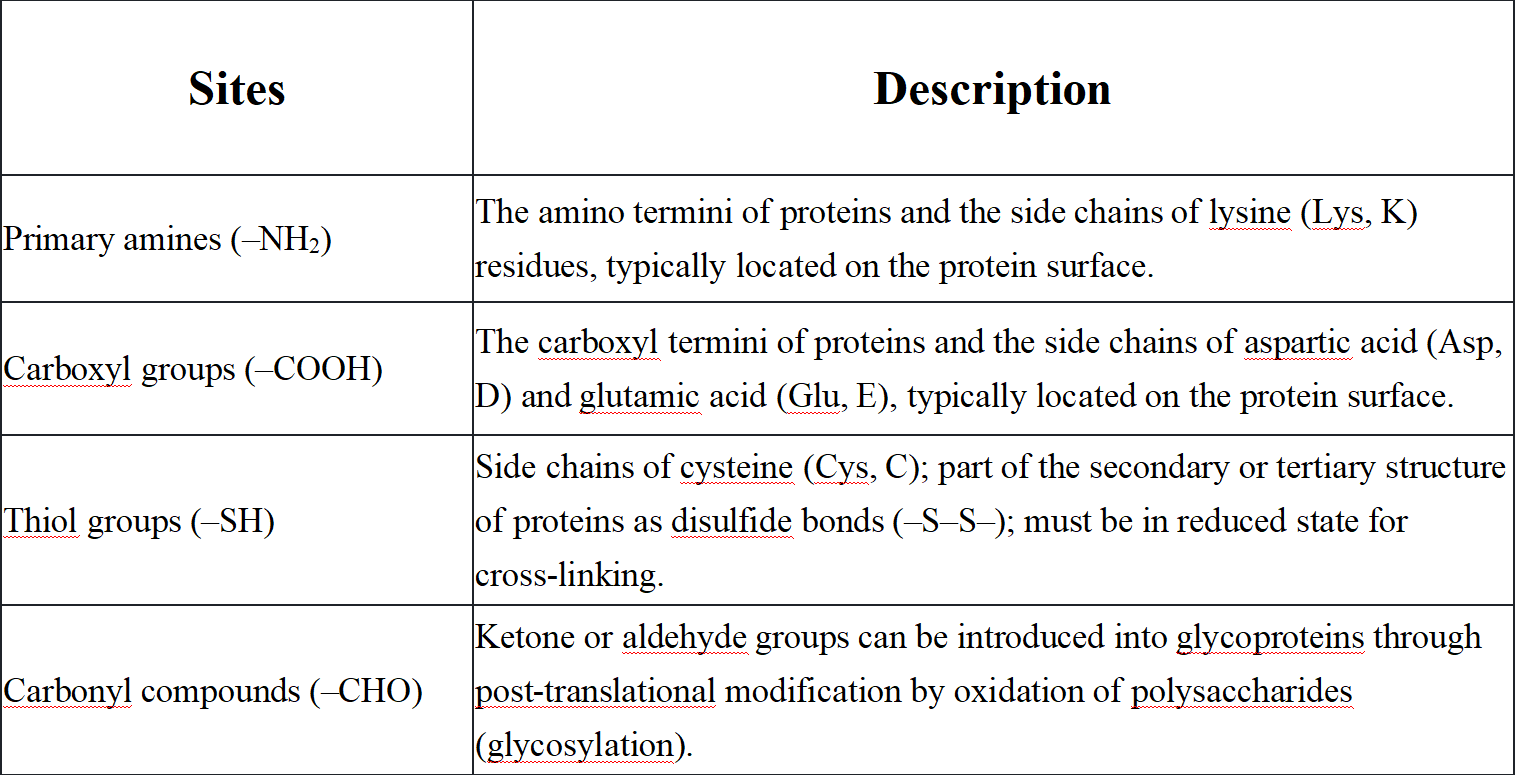
Antibody labeling typically employs one of four attachment methods: connecting through charged amino acids, attaching to sugar molecules (carbohydrates), linking via sulfur-containing cysteine residues, or attaching to tyrosine residues. The primary sites for labeling or cross-linking biomolecules (especially proteins) on antibodies are the charged amino acids on the external surface of the antibody. Primary amines are typically the target for coupling chemistry due to their abundance, widespread distribution, and ease of modification due to their reactive nature. The second target for antibody labeling is the carbohydrate molecules on the highly glycosylated Fc portion of the antibody. One advantage of labeling at these sites is their distance from the antigen-binding sites, reducing the likelihood that labeling interferes with antigen binding. The third target for antibody labeling is the thiol groups in cysteine residues. Cysteine can be reduced (freed) or oxidized (forming disulfide bonds between two cysteines). Tyrosine residues in antibodies can be radioactively labeled through specific iodination.
Many antibody labeling methods utilize chemical cross-linkers to facilitate the addition of desired modifications. Important considerations include the chemical specificity of the cross-linker for certain functional groups and potential specific side reactions, whether the cross-linker should be hetero- or homo-bifunctional, the length of the spacer arm between the two linking parts, and the solubility of the cross-linker in water or appropriate organic solvents.
Depending on the type of ELISA being developed, antigens or antibodies are generally labeled with horseradish peroxidase (HRP) or biotin. Biotinylation of antibodies (biotin labeling) is a useful and simple technique. The small size of biotin (244 Da) typically has minimal impact on the biological activity of protein targets. Biotin binds to its binding partners, streptavidin, and avidin, with very high affinity (Kd ≈ 10−15 mol/L) and specificity. Modified forms of streptavidin and avidin, whether labeled themselves or conjugated with proteins, enzymes, or used in combination with dyes or other detection reagents, can be used with biotinylated antibodies to significantly increase the reagent's versatility and amplify the output signal. Peroxidase is a heme-containing oxidoreductase enzyme of significant commercial value, capable of catalyzing hydrogen peroxide from electron donors. The HRP enzyme, as an antibody-enzyme conjugate, has been used for over 40 years due to its ease of covalent attachment and retention of high enzymatic activity, making it the preferred choice. The most common applications of HRP conjugates include western blotting, ELISA, and immunohistochemistry. HRP conjugates are widely recognized and used in biological applications due to their adaptability, small size, stability, relative features, and lower cost.
2. Optimization of Antigen and Antibody Concentration and Reaction Conditions
Evaluate the sensitivity and specificity of antigen-antibody reactions at varying concentrations to determine the optimal concentrations of antigens and antibodies for use. Determine coating buffer, washing buffer and enzyme conjugate storage solution. Additionally, establishing the optimal temperature and duration for antibody reactions is also essential.
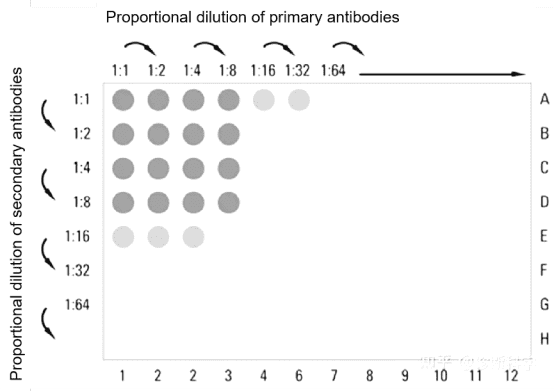
Figure 3. Elisa Antibody Concentration Optimization
3. Quality Assessment of Labeled Antibodies
(1) Evaluating Recovery Rates
Recovery rates assess whether the sample matrix affects the detection of target substances. Calculated as (detected value of the target substance in a matrix-diluted sample) / (actual value of the matrix-diluted target substance) * 100%, an acceptable range is 80%-120%. Deviations outside this range indicate significant matrix-related experimental interference.
(2) Determining Sensitivity (Detection Limit)
Sensitivity refers to the lowest level of analyte that can be distinguished from background, often called analytical sensitivity or detection limit. It's quantified through three metrics: Limit of blank (LOB) refers to the highest measurement outcome achievable with blank samples under a specified probability; limit of detection (LOD) is defined as the lowest value that can be reliably measured under a certain probability, ensuring that the presence of an analyte is detectable; and limit of quantitation (LOQ) is commonly referred to as the limit of quantitation. This is also a lower value obtained by testing a low-concentration analyte sample. The accuracy of this value is required, that is, it must not only be detectable, but also make sure the results are accurate.
(3) Assessing Reproducibility (Inter and Intra-Assay Variability)
Reproducibility is gauged by the coefficient of variation (CV), the ratio of standard deviation to the mean, across different assay wells. Ideally, the %CV should be below 10%, indicating consistent results with minimal variability.
(4) Stability Analysis
Stability is tested through accelerated degradation at 37 degrees over various periods (3, 7, 10, 14 days), with parallel long-term assessments at 4 ℃. Generally, the test kit can be stored at 4 ℃ for 1 year.
(5) Examining Dilution Linearity
This process evaluates the accuracy of test results across a spectrum of dilution levels, expecting a 70%-130% match between measured and actual values. This criterion helps identify and mitigate matrix interference effects on sample analysis.
(6) Signal/Noise Assessment:
Signal/Noise (S/N) is the ratio of signal to noise, that is, the ratio of sample data to blank data. A 3:1 ratio typically defines the detection limit, while a 10:1 ratio sets the quantitation threshold, ensuring clarity and reliability in results.
Analysis Workflow
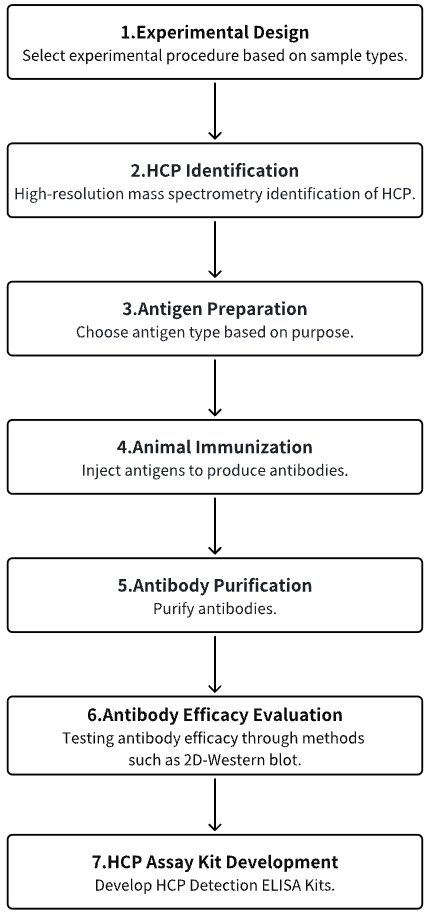
1. Experimental Procedure Determination Based on Experimental Requirements
2. HCP Identification
3. Host Cell Antigen Preparation
4. Animal Immunization
5. Antibody Purification
6. Antibody Efficacy Evaluation
7. HCP Assay Kit Development
Service Advantages
1. High Reliability Identification and Characterization of HCPs
2. Multiple Antigen Preparation Platforms
3. Comprehensive and Strict Immunization Procedures
4. Effective Antibody Detection Systems and Extensive Detection Experience
Sample Results
1. CHO HCPs Platform: A Promising Method for Monitoring HCPs via ELISA
HCPs, major process-related impurities originating from host organisms, can influence the quality, safety, and efficacy of products, even in minute quantities. Thus, optimizing the product purification process to remove as many HCPs as feasible, aiming for the highest purity, is crucial. The sandwich ELISA, employing polyclonal antibodies against HCPs for both capture and detection, is a primary method for HCP monitoring and quantification in bioprocessing. Commercial ELISA kits, developed using CHO cell lines and employed in early drug development stages (preclinical, phase I, and II), are not tailored for proprietary cell lines of specific manufacturers, limiting control over reagent availability and consistency. For later development stages, upstream process-specific methods, offering greater sensitivity and coverage, should be prioritized. According to the US Pharmacopeia general chapter <1132>, if a platform assay is available at product development's outset, it can replace commercial assays throughout all development phases. Studies aimed to showcase the feasibility and benefits of creating a proprietary CHO HCPs platform ELISA have been conducted. Using orthogonal two-dimensional electrophoresis techniques (SDS-PAGE coupled with SS/WB and 2D DIGE), proprietary mock materials were characterized and compared to identify optimal antigen-antibody pairs for internal ELISA construction. Preliminary evaluations of the internal method, compared to commercial methods, suggest the platform's potential for accurate early-stage quantification of CHO HCPs in product and process development.
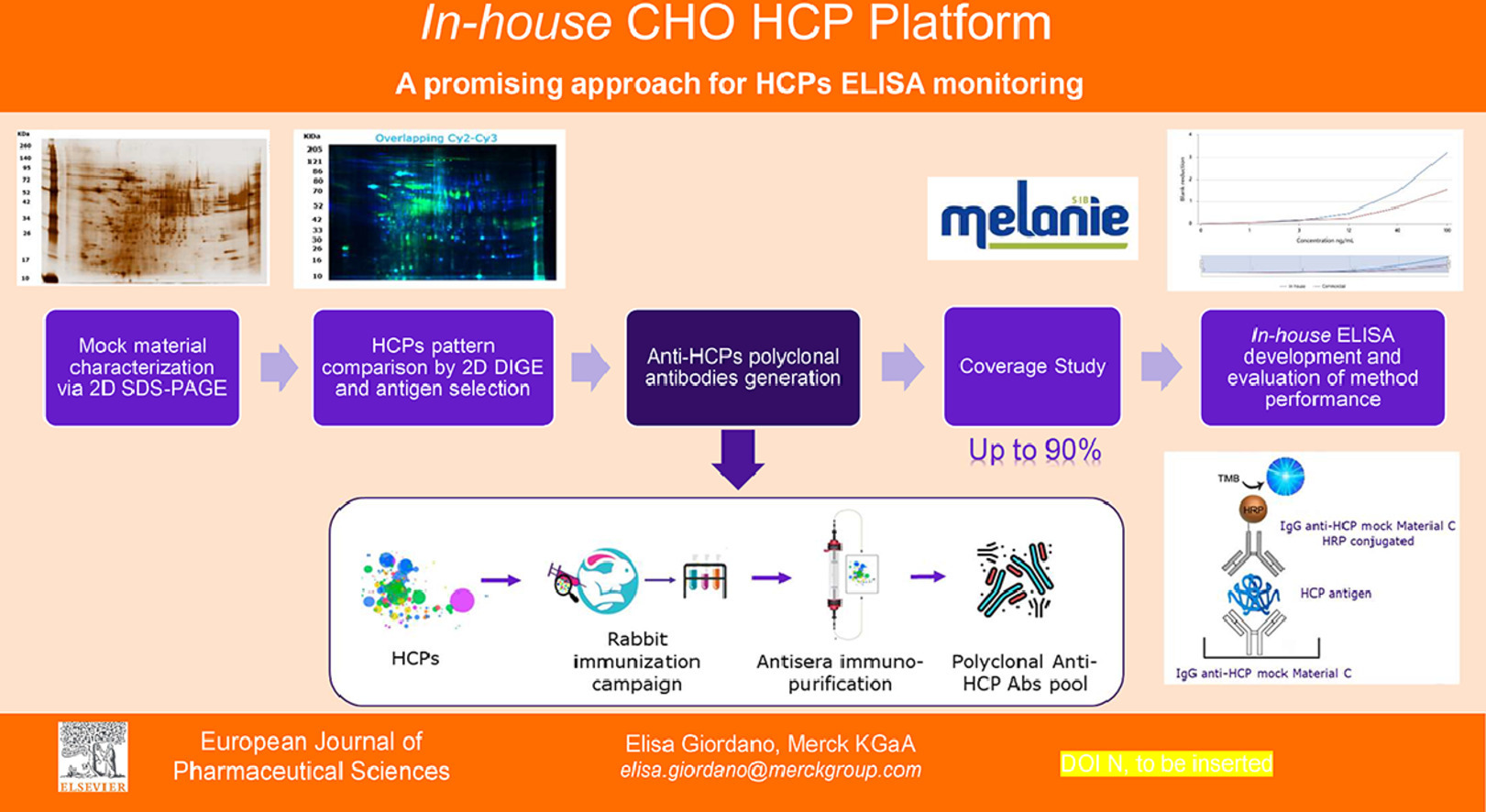
Figure 4. CHO HCPs Platform Schematic [5]
2. Comparing Platform HCP ELISA with Process-Specific HCP ELISA
During the expression of therapeutic proteins, the normal expression mechanisms of host cells generate a complex mixture of other proteins. HCPs, posing potential risks to patient safety and product efficacy, require thorough characterization and demonstration of their clearance from the process. The complexity of HCPs necessitates demonstrably effective monitoring methods to adequately inform about relevant proteins. ELISA is the most commonly used analytical method for HCP monitoring. Ensuring the development of a suitable HCP ELISA hinges on the careful selection of key reagents, including anti-HCP antibodies and analytical standards. Recent significant updates to biopharmaceutical drug production processes prompted a reevaluation of the suitability of existing HCP ELISAs for monitoring HCP populations during updates. This evaluation involved comparing process-specific ELISAs with platform ELISAs. Despite qualitative differences in HCP spectra observed in 2D PAGE, LC-MS/MS revealed similar HCP populations within both standards. Process-specific HCP antibodies demonstrated adequate coverage but heightened sensitivity to a few dominant proteins present in the upstream purification process. In contrast, platform HCP antibodies offered broad coverage, detecting most potential HCP impurities, and showed no bias toward dominant proteins, proving more sensitive in downstream purification processes. Given its extensive HCP coverage and sensitivity, the study concludes that our platform HCP ELISA method outperforms the process-specific HCP ELISA method.
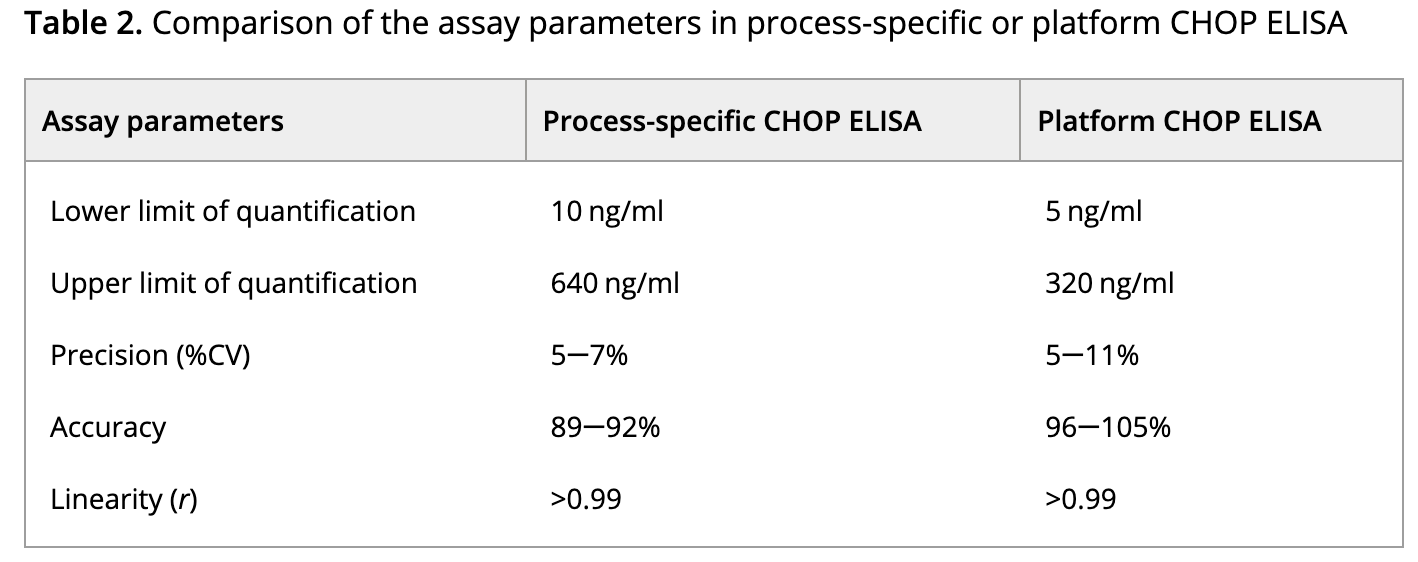
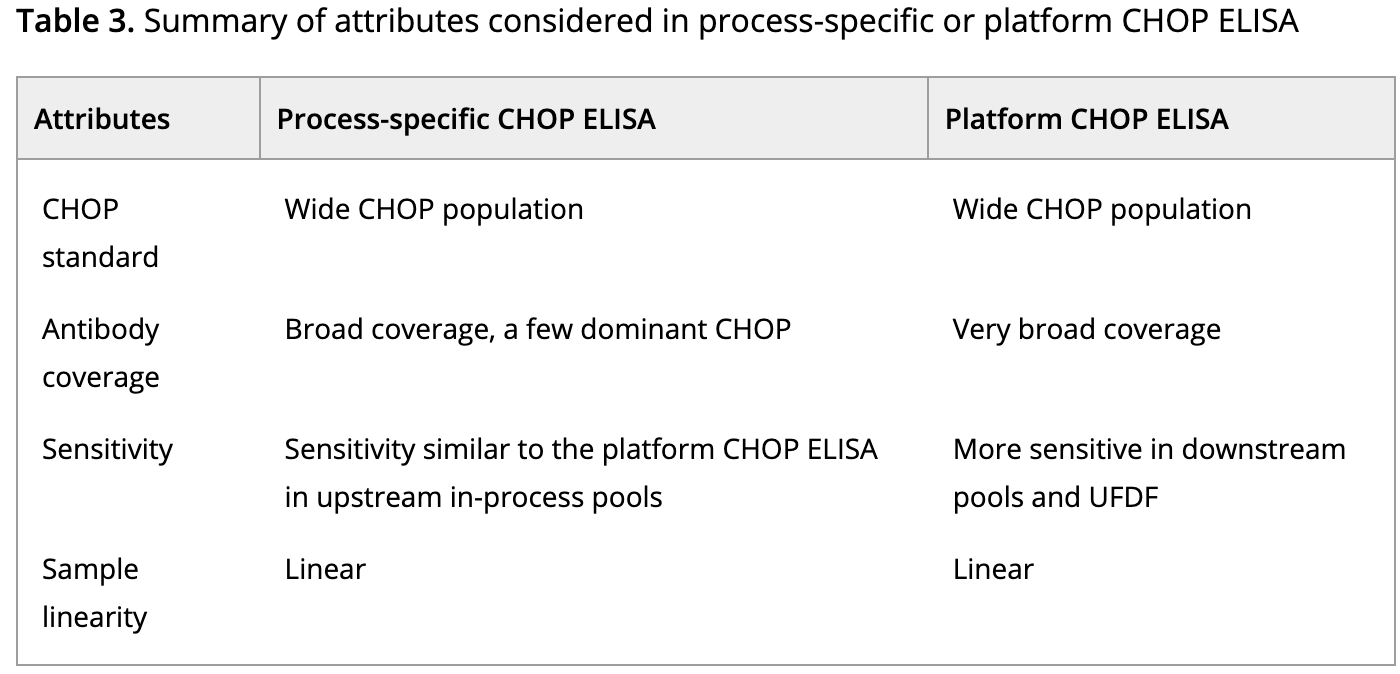
Figure 5. Comparison of Assay Parameters in Process-Specific or Platform CHOP ELISA [6]
3. The Impact of Host Animal Species on the Performance of Quantitative HCP ELISA
HCPs are inevitable process-related impurities in biopharmaceutical drugs, typically monitored through ELISA. Polyclonal anti-HCP antibodies (pAbs) are crucial for their reliable detection as they should be capable of recognizing a broad range of HCPs. This study aimed to identify the host animal species suitable for developing a high-performance CHO-HCP ELISA, assuming the generation of pAbs with ample coverage and specificity. Comparing antibodies immunized in sheep, goats, donkeys, rabbits, and chickens in sandwich ELISAs elucidated the quantity, coverage, and performance of HCP-specific antibodies. Immunizations in sheep, goats, donkeys, and rabbits met all test standards, while chicken-derived antibodies were not recommended based on this study. Moreover, antibody mixtures from the five host species were prepared to assess if a multi-species approach could enhance coverage and ELISA performance. Comparable results between single-species and multi-species ELISAs across different process samples indicated no substantial improvement in ELISA performance with the latter approach, raising ethical and financial concerns.
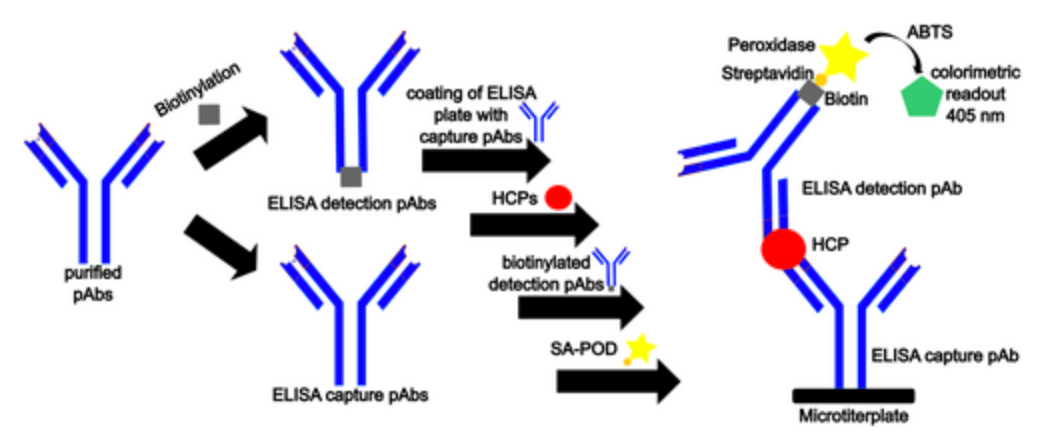
Figure 6. Illustration Using Sandwich ELISA [7]
Sample Submission Requirements
1.Minimization of Impurity Contamination
Services at MtoZ Biolabs
1. Experimental Step Completion
2. Instrument Parameter Relevance
3. Original Experimental Data
4. Data Analysis Submission
FAQ
Q1: What are the common cross-linking sites on antibodies?
References
[1] Tuameh A, Harding SE, Darton NJ. Methods for addressing HCPimpurities in biopharmaceutical product development. Biotechnol J. 2023 Mar;18(3):e2200115. doi: 10.1002/biot.202200115. Epub 2022 Dec 13. PMID: 36427352.
[2] Haltaufderhyde K, Roberts BJ, Khan S, Terry F, Boyle CM, McAllister M, Martin W, Rosenberg A, De Groot AS. Immunoinformatic Risk Assessment of HCPs During Process Development for Biologic Therapeutics. AAPS J. 2023 Sep 11;25(5):87. doi: 10.1208/s12248-023-00852-z. Erratum in: AAPS J. 2023 Dec 19;26(1):6. PMID: 37697150.
[3] Pilely K, Johansen MR, Lund RR, Kofoed T, Jørgensen TK, Skriver L, Mørtz E. Monitoring process-related impurities in biologics-HCPanalysis. Anal Bioanal Chem. 2022 Jan;414(2):747-758. doi: 10.1007/s00216-021-03648-2. Epub 2021 Oct 1. PMID: 34595561; PMCID: PMC8483941.
[4] Ahirwar R, Bhattacharya A, Kumar S. Unveiling the underpinnings of various non-conventional ELISA variants: a review article. Expert Rev Mol Diagn. 2022 Jul;22(7):761-774. doi: 10.1080/14737159.2022.2117615. Epub 2022 Sep 7. PMID: 36004453.
[5] Giordano E, Liori B, Cecchini I, Verani R, Leone L. In-house CHO HCPs platform: A promising approach for HCPs ELISA monitoring. Eur J Pharm Sci. 2024 Jan 1;192:106656. doi: 10.1016/j.ejps.2023.106656. Epub 2023 Nov 27. PMID: 38029932.
[6] Gunawan F, Nishihara J, Liu P, Sandoval W, Vanderlaan M, Zhang H, Krawitz D. Comparison of platform HCPELISA to process-specific HCPELISA. Biotechnol Bioeng. 2018 Feb;115(2):382-389. doi: 10.1002/bit.26466. Epub 2017 Oct 30. PMID: 28986978.
[7] Seisenberger C, Graf T, Sticht S, Haindl M, Mohn U, Wegele H, Wiedmann M, Wohlrab S. The agony of choice: Impact of the host animal species on the enzyme-linked immunosorbent assay performance for HCPquantification. Biotechnol Bioeng. 2023 Jan;120(1):184-193. doi: 10.1002/bit.28265. Epub 2022 Nov 8. PMID: 36251621.
MtoZ Biolabs, an integrated chromatography and mass spectrometry (MS) services provider.
Related Services
Host Cell Protein (HCP) Detection Service
How to order?







