From Qualitative to Quantitative: The Comprehensive Capabilities of Mass Spectrometry in PTM Research
-
High sensitivity: Capable of detecting low-abundance modified peptides
-
High specificity: Able to identify precise modification sites
-
High throughput: Suitable for analyzing thousands of modification events simultaneously
-
High dynamic range: Effective for a wide range of sample types and processing conditions
-
Labeling methods (e.g., TMT/iTRAQ): These methods support multiple sample parallelism, with high reproducibility, making them ideal for systematic studies.
-
Label-free methods: These are simpler and more cost-effective, making them suitable for exploratory analyses.
Post-translational modifications (PTMs) play a pivotal role in the regulation of various biological processes, including signal transduction, metabolic regulation, epigenetics, and immune responses. As proteomics technologies have evolved, mass spectrometry (MS) has emerged as a critical tool for analyzing PTMs. Initially used for qualitative detection, MS has now progressed to high-throughput, reproducible quantitative analysis, demonstrating unparalleled capabilities in PTM research.
Why Is Mass Spectrometry Essential for PTM Research?
The central challenge in PTM research lies in the diversity, low abundance, and dynamic nature of these modifications. Common modifications such as phosphorylation, acetylation, ubiquitination, methylation, and glycosylation typically occur in low quantities at specific sites and can rapidly change in response to external stimuli. Conventional protein detection methods have limitations in throughput and site-specific resolution, making it difficult to meet the needs for systematic and high-resolution modification studies. Mass spectrometry, however, offers several advantages that make it an essential tool in PTM research:
From Qualitative to Quantitative: The Three Capabilities of Mass Spectrometry in PTM Research
1. Precise Identification: High Confidence in Modifications Site Identification
In the initial stages of PTM research, scientists typically focus on determining whether a modification has occurred and where the modification sites are located. At this stage, mass spectrometry is primarily used for identifying modified peptides and pinpointing the exact modification sites. By combining enzymatic digestion, enrichment techniques, and high-resolution mass spectrometry, a variety of modifications can be detected, with precise locations determined through database searches. Optimized enrichment strategies, such as TiO₂ or antibody-based enrichment, in conjunction with tandem mass spectrometry (MS/MS), significantly improve the sensitivity of detecting modified peptides. These methods offer considerable advantages, especially for studying low-abundance modifications like phosphorylation and acetylation.
2. Relative Quantification: Comparison of Modification Levels under Different Conditions
Understanding not only whether a modification occurs, but also the extent of the modification, is crucial in PTM research. Relative quantification is essential for examining dynamic PTM changes during mechanistic studies, drug screening, or disease model creation. Common strategies for relative quantification include:
By statistically analyzing the intensities of modification peptides under various conditions, scientists can trace the dynamic fluctuations of PTM during processes like signal pathway activation, transcription factor regulation, or epigenetic changes.
3. Absolute Quantification: Constructing PTM Quantification Reference Maps
In areas such as drug development and biomarker validation, relative quantification alone is no longer sufficient to meet the need for precise measurements. Absolute quantification is becoming an increasingly important focus in PTM research. This approach typically involves using synthetic peptide standards and targeted mass spectrometry techniques, such as Parallel Reaction Monitoring (PRM) or Multiple Reaction Monitoring (MRM), to precisely measure the concentration of specific modified peptides.
Mass Spectrometry Strategies for Common PTM Types
Different types of modifications exhibit unique characteristics in terms of their chemical structure, abundance, and enrichment methods. Therefore, mass spectrometry strategies must be tailored to each modification type.
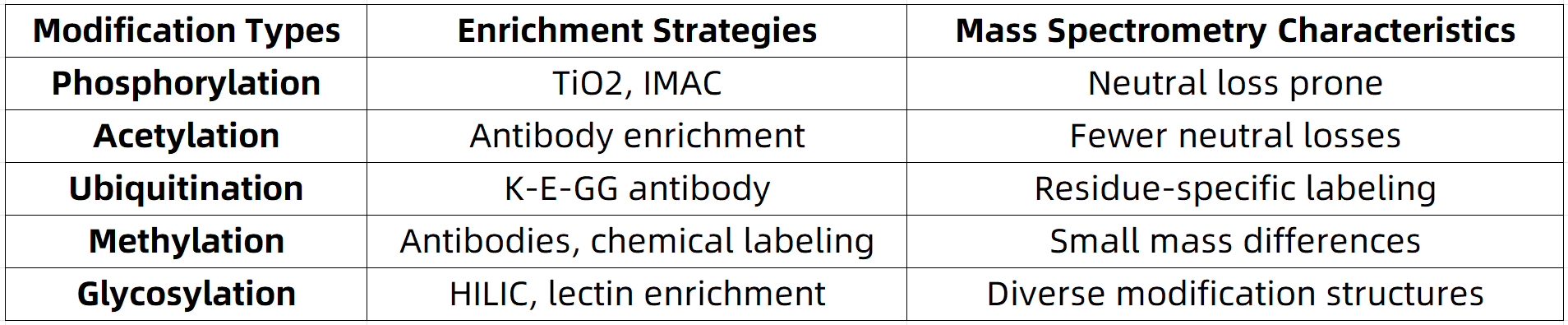
Through the optimization of sample preparation, enrichment condition control, scan mode adjustment, and advanced data analysis, mass spectrometry can precisely meet the diverse needs of PTM research.
Overcoming Data Barriers: The Trend of Integrated PTM Omics Analysis
As systems biology rapidly advances, relying solely on a single dimension of PTM analysis is no longer sufficient for a comprehensive understanding of complex biological processes. An increasing number of research teams are adopting multi-PTM collaborative analysis strategies, integrating data from modifications such as phosphorylation, acetylation, and ubiquitination to explore their interdependent regulatory networks. Additionally, integrating PTM omics with other omics data such as proteomics, interactomics, transcriptomics, and metabolomics for joint analysis can significantly enhance biological interpretation and accelerate the discovery of functional mechanisms.
Throughout the entire process of protein post-translational modification research, from the initial identification of modifications, to the comparative analysis of conditions, and finally to the quantitative validation of targets, mass spectrometry continues to play a pivotal role. Its sensitivity, high throughput, and quantitative capabilities make it the "gold standard" tool in both fundamental and precision medicine research. MtoZ Biolabs focuses on the advanced application of mass spectrometry in PTM research, offering comprehensive solutions that include modification type screening, sample pretreatment, selection of quantitative strategies, data mining, and biological annotation to assist researchers in thoroughly exploring PTM regulatory mechanisms and accelerating scientific breakthroughs.
MtoZ Biolabs, an integrated chromatography and mass spectrometry (MS) services provider.
Related Services
How to order?







