Freezing Point Osmometry Analytical Service
- Vibration method: Induces crystallization through mechanical vibration, reducing contact contamination and providing stable results.
- Two-needle method: Induces crystallization by direct contact with metal probes, a proven and broadly applied approach that suits diverse testing requirements.
Freezing Point Osmometry is a robust analytical technique used to determine osmolality by measuring the degree of freezing point depression in a solution. Since osmolality reflects the number of solute particles rather than their molecular weight, it provides a reliable measure of solute concentration in complex systems. This approach is widely applied in pharmaceutical development, clinical diagnostics, and food and beverage testing.
MtoZ Biolabs offers high-quality Freezing Point Osmometry Analytical Service supported by advanced instruments and an experienced scientific team. We deliver fast, accurate, and reproducible results that empower both academic research and industrial applications.
Technical Principles
Freezing Point Osmometry is based on the law of freezing point depression. Solute particles interfere with solvent crystallization, causing the freezing point of the solution to drop below that of the pure solvent. The extent of freezing point depression is directly proportional to the solute particle concentration. By precisely recording the freezing curve of a sample and applying this principle, the osmolality of the solution can be calculated with high accuracy.
Two widely used crystallization induction methods include:
MtoZ Biolabs applies the most appropriate method depending on sample type and research objectives, ensuring reliable outcomes.
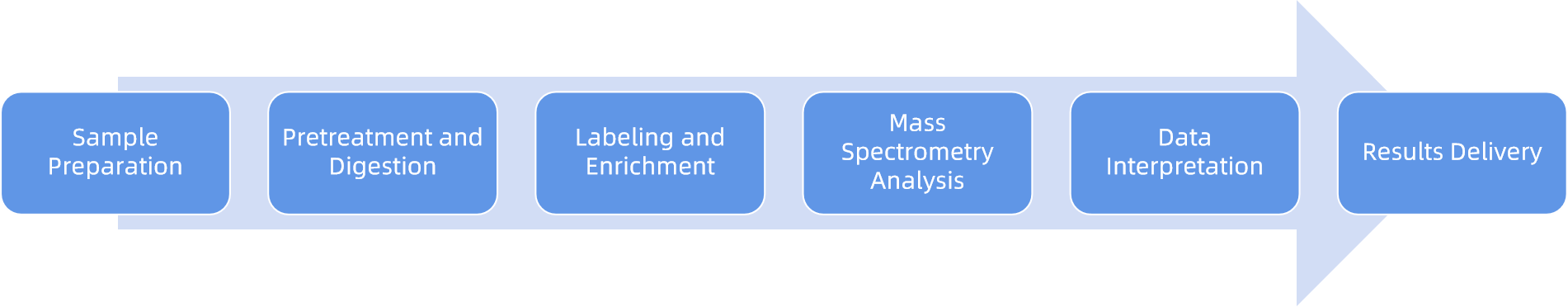
Analysis Workflow
To guarantee reproducibility and accuracy, MtoZ Biolabs follows a standardized workflow:
1. Sample Pretreatment: Clarification and removal of impurities from serum, urine, drug formulations, or culture media.
2. Crystallization Induction: Triggering crystallization using vibration or two-needle methods under controlled conditions.
3. Freezing Point Detection: Monitoring freezing events in real time using high-precision sensors.
4. Osmolality Calculation: Converting freezing point depression data into osmolality values.
5. Data Analysis and Quality Control: Applying cross-validation and calibration to confirm reliability.
6. Reporting: Delivering a detailed report with experimental conditions, results, and professional interpretation.
Sample Submission Suggestions
We accept a wide range of samples including purified solutions, pharmaceutical formulations, buffers, and biological fluids such as blood, urine, and tissue homogenates. Samples may be submitted in liquid or lyophilized form. For complex or unusual matrices, clients are encouraged to consult our team prior to submission. MtoZ Biolabs provides a comprehensive submission guide to ensure accurate and reproducible results.
Why Choose MtoZ Biolabs?
✅ High-Sensitivity Detection: Advanced osmometers enable precise analysis with minimal sample volumes.
✅ Versatile Method Options: Both vibration and two-needle methods are available to suit different sample types.
✅ Broad Applicability: Compatible with pharmaceuticals, clinical fluids, bioproducts, and culture media.
✅ Strict Quality Assurance: Parallel sample testing and internal standards guarantee reproducibility and reliability.
Applications
Freezing Point Osmometry Analytical Service supports diverse research and industrial needs, including:
● Pharmaceutical Development: Validating stability and formulation consistency of injectables, ophthalmic solutions, and oral liquid drugs.
● Biopharmaceutical Quality Control: Monitoring osmolality variations during vaccine, antibody, or culture media production.
● Food and Nutrition Research: Determining solute concentration and product stability in beverages and nutritional solutions.
● Basic Research: Investigating cellular osmoregulation mechanisms and environmental stress responses.
What Could be Included in the Report?
1. Experimental details and methodology
2. Materials, instruments, and workflow documentation
3. Freezing curve data with quality control assessment
4. Osmolality values with statistical analysis
5. Optional bioinformatics support
5. Raw data files
FAQ
Q1: What advantages does Freezing Point Osmometry have over other osmolality measurement methods?
Freezing Point Osmometry is based on the physical principle of freezing point depression and directly reflects the total number of solute particles in a solution. It does not depend on solute type, which ensures broad applicability and high reliability in complex systems such as multi-component drugs or biological fluids.
MtoZ Biolabs’ Freezing Point Osmometry Analytical Service integrates advanced instrumentation, standardized workflows, and expert interpretation to deliver high-quality, reproducible osmolality testing. Our service supports pharmaceutical R&D, clinical studies, food science, and basic research. Contact MtoZ Biolabs today to discuss your project and receive a customized testing plan tailored to your research goals.
Related Services
How to order?







