Fmoc Solid Phase Peptide Synthesis Service
Fmoc Solid Phase Peptide Synthesis Service is based on solid support synthesis of peptides by sequentially coupling amino acids in a specific order to synthesize peptides with defined sequences and controllable structures. Unlike traditional solution-phase synthesis, Fmoc Solid Phase Peptide Synthesis (Fmoc-SPPS) uses solid-phase resin to immobilize the peptide chain, making the separation and washing of reactants more efficient.
Before each amino acid addition, the Fmoc group protects its α-amino group, preventing nonspecific reactions. After deprotection using piperidine solution to expose the active amino group, coupling reagents facilitate the formation of peptide bonds between the amino and carboxyl groups, ensuring specificity at every step. Finally, the synthesized peptide is cleaved from the resin using trifluoroacetic acid (TFA), which also removes side-chain protecting groups, yielding the target peptide. The process of Fmoc-SPPS minimizes side reactions and supports the introduction of complex functional modifications such as fluorescent labeling, phosphorylation, or cyclization.
With extensive experience in peptide synthesis and advanced experimental platforms, MtoZ Biolabs offers Fmoc Solid Phase Peptide Synthesis Service for synthesizing short peptides, modified peptides, cyclic peptides, and other complex peptide molecules.
Analysis Workflow
General workflow for Fmoc Solid Phase Peptide Synthesis Service:
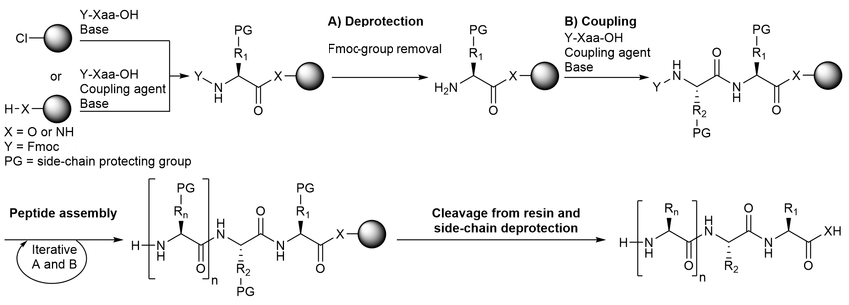
Bédard F. et al. Frontiers in Microbiology. 2018.
1. Resin Preparation
Select the appropriate resin based on the target peptide sequence, and perform swelling and activation.
2. Amino Acid Coupling
Mix Fmoc-protected amino acids with an activator and add them to the resin to form peptide bonds.
3. Fmoc Deprotection
Use piperidine solution to remove the Fmoc group, exposing the free amine for subsequent coupling.
4. Repeated Coupling and Deprotection
Repeat the coupling and deprotection steps according to the sequence to extend the peptide chain step by step.
5. Peptide Cleavage and Deprotection
Use a strong acidic reagent (e.g., TFA) to cleave the peptide from the resin and simultaneously remove side-chain protecting groups.
6. Purification and Validation
Purify the target peptide using high-performance liquid chromatography (HPLC) and verify its molecular weight with mass spectrometry to ensure successful synthesis.
Applications
Common applications of Fmoc Solid Phase Peptide Synthesis are as follows:
Drug Development
Synthesis of antimicrobial peptides, antitumor peptides, hormone peptides, antiviral peptides, and more to support the development of novel drugs.
Structure and Function Studies
Preparation of modified peptides, cyclic peptides, or functional peptide fragments for research into protein structure and functional mechanisms.
Biomarker Development
Synthesis of biomarker peptide fragments for early disease detection or immunodiagnostics.
Vaccine Research
Production of antigen peptides for vaccine design, immune response analysis, or antibody generation.
FAQ
Q. How to Avoid Low Yield and Side Reactions in Long Peptide (>30 Amino Acids) Synthesis?
Synthesizing long peptides presents challenges such as decreased coupling efficiency, accumulation of side reactions, and peptide chain aggregation hindering elongation. The following strategies can effectively improve the success rate and yield of long peptide synthesis:
1. Optimize Coupling Efficiency
Use high-efficiency coupling agents: Employ reagents such as HATU to significantly enhance coupling efficiency and reduce the likelihood of side reactions.
Extend coupling time: Allow longer reaction times for each amino acid coupling step to ensure reaction completion.
Double coupling strategy: Perform double coupling for each amino acid to further minimize the risk of incomplete coupling.
2. Use Cosolvents to Reduce Aggregation
During long peptide synthesis, intra-chain hydrogen bonding can lead to peptide chain aggregation. Adding cosolvents like dimethyl sulfoxide (DMSO) can disrupt aggregation structures, improving elongation efficiency.
3. Select Appropriate Resin
Use low-loading resins (e.g., 0.2–0.4 mmol/g) to reduce peptide chain density, mitigating inter-chain interactions and aggregation risks.
4. Stepwise Cleavage Strategy
Side-chain protecting groups on long peptides may lead to complex byproducts during final cleavage. A stepwise cleavage strategy, removing protecting groups in phases, can minimize side reactions.
5. Strictly Control Reaction Conditions
Regularly monitor the completion of coupling and deprotection steps to ensure each reaction is carried out efficiently.
6. Fragment Assembly Approach
For ultra-long peptides, employ a peptide fragment condensation strategy, chemically joining shorter fragments to form the full-length peptide chain.
Case Study
This study successfully synthesized a light-controlled Caspase inhibitory peptide using Fmoc solid phase peptide synthesis technology, providing a novel research tool for precise regulation of apoptosis.
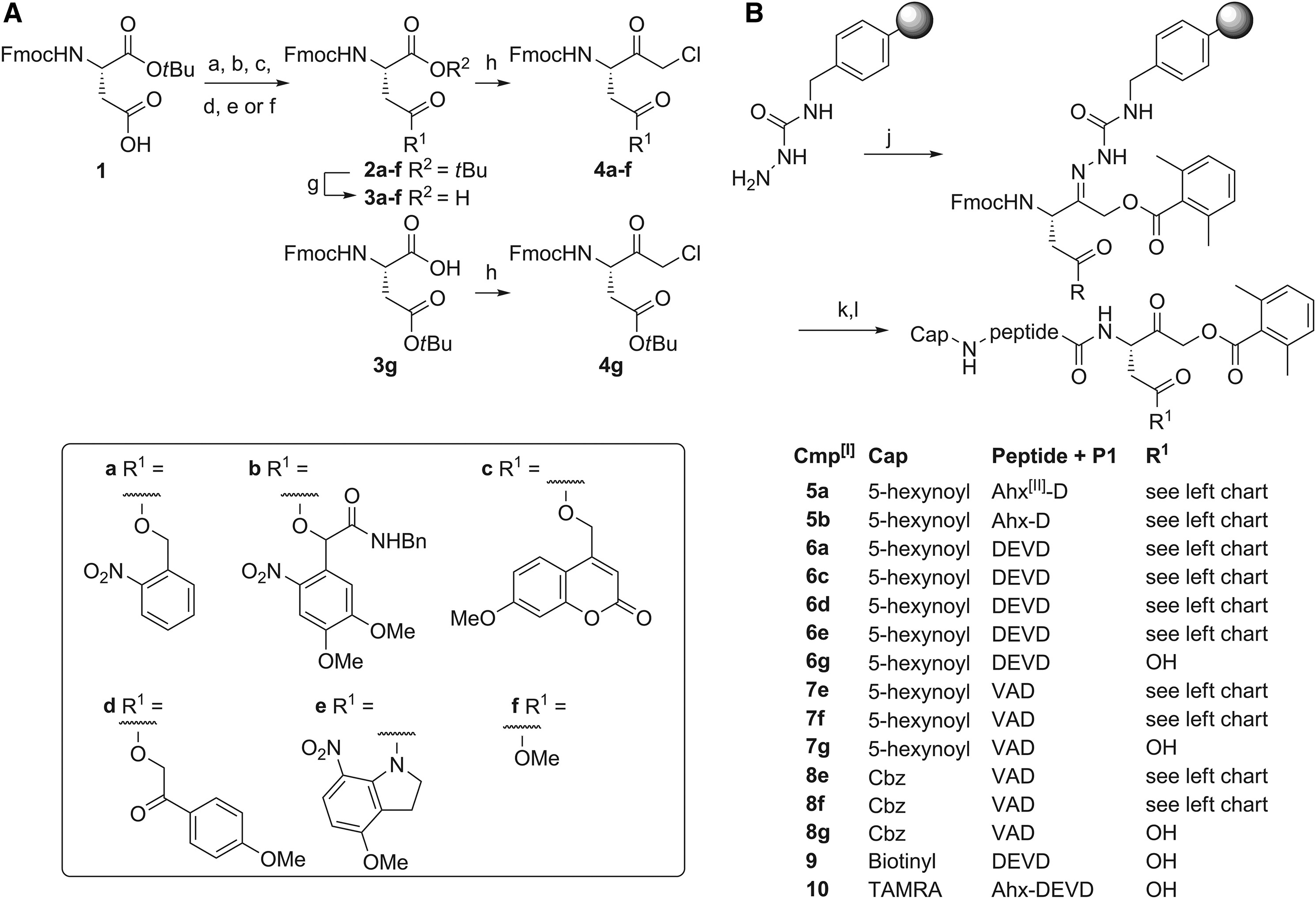
Chakrabarty S. et al. Cell Chemical Biology. 2020.
MtoZ Biolabs, an integrated chromatography and mass spectrometry (MS) services provider.
Related Services
Verification Service of Synthetic Peptide Sequence
How to order?







