Exosome Studies Service
- Cancer Metabolism Research: Investigate the metabolic roles of exosomes in tumor microenvironments.
- Biomarker Discovery: Identify characteristic metabolites associated with diseases, including cancer and metabolic disorders.
- Personalized Medicine: Utilize exosomal profiles to inform tailored treatment strategies.
- Tissue Repair Mechanisms: Explore the potential of exosomes in regenerative medicine.
Exosomes are small vesicles, ranging from 30 to 150 nm in diameter, secreted by live cells. These extracellular vesicles contain a complex mixture of biomolecules, including proteins, nucleic acids, and lipids, derived from the originating cells. Present in a variety of body fluids such as blood, urine, and saliva, exosomes represent a novel intercellular communication system that significantly impacts cellular physiology and is closely associated with the development and progression of various diseases.
Exosomes play a crucial role in intercellular signaling, influencing processes such as immune responses, antigen presentation, cell migration, differentiation, and tumor invasion. Tumor-derived exosomes, in particular, facilitate the transfer of genetic information between cancer cells and surrounding cells. Their applicability extends to biomarker screening, drug delivery systems, disease therapy, and physiological regulation. Moreover, exosomes exhibit functions similar to those of stem cells in tissue repair, making them valuable in regenerative medicine and increasingly popular in the aesthetics industry due to their immunogenic properties and ease of absorption. The integration of omics technologies—such as transcriptomics, proteomics, and metabolomics—provides a comprehensive understanding of the biomolecular landscape of exosomes. This approach can uncover new mechanisms underlying human diseases, identify biomarkers, and highlight potential therapeutic targets.
MtoZ Biolabs employs advanced methodologies for exosome studies, utilizing various extraction techniques, including ultracentrifugation, polymer precipitation, and innovative TiO2 enrichment methods. Each method has its advantages and limitations, allowing us to choose the most suitable approach for specific research needs.
Analysis Workflow

Service Advantages
1. High Sensitivity and Accuracy
Our advanced mass spectrometry technologies ensure effective identification of low-abundance biomolecules.
2. Comprehensive Analysis
We cover multiple classes of biomolecules, providing robust data to support your research.
3. Rapid Turnaround
Our streamlined processes enable us to deliver high-quality analysis results promptly.
4. Expert Technical Support
Our experienced team offers personalized consultation and guidance tailored to your specific research objectives.
Sample Submission Requirements
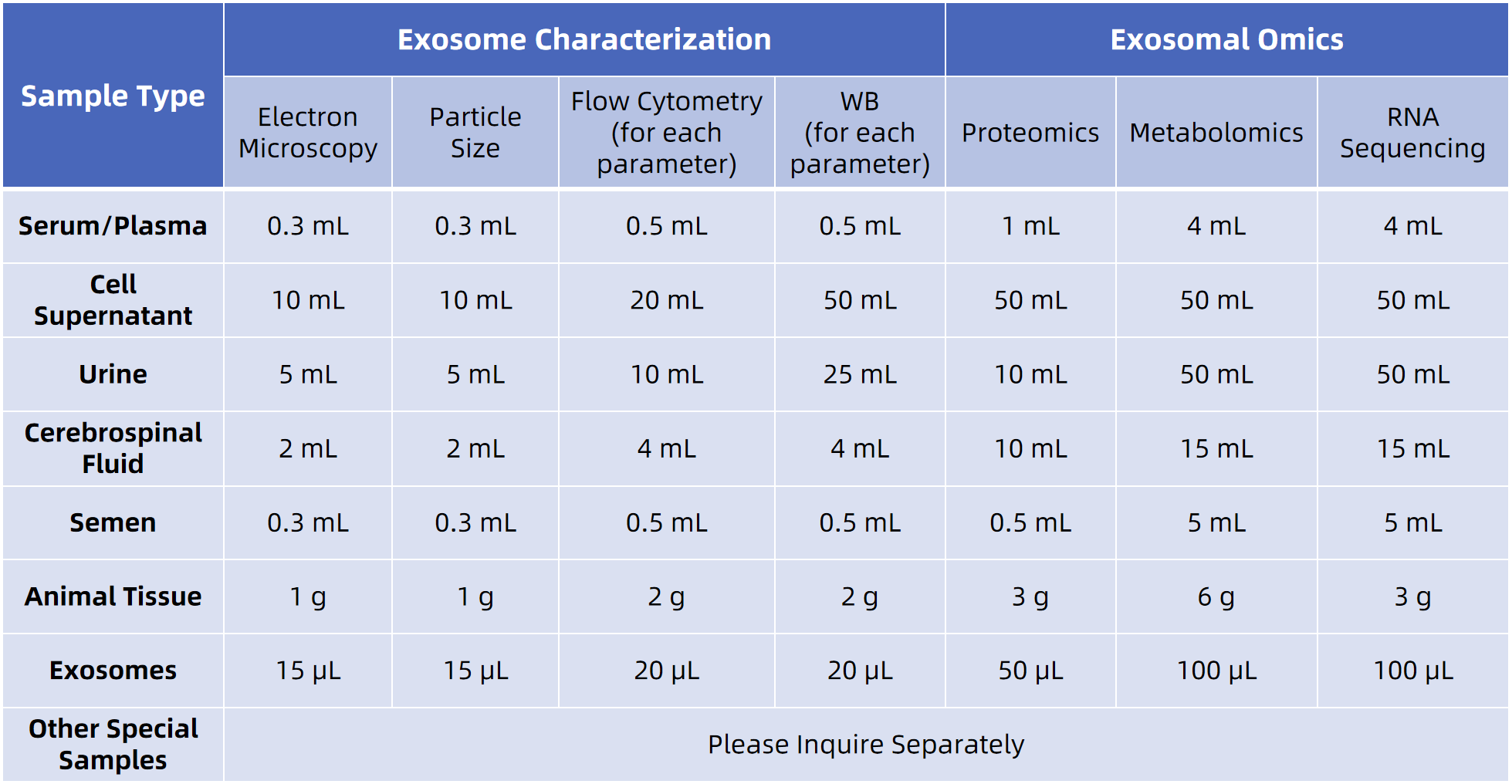
For more sample details, please consult our technical team.
Applications
How to order?







