Exosome Quality Control Service
- Transmission Electron Microscopy (TEM) – Provides high-resolution imaging of exosome ultrastructure.
- Cryo-Electron Microscopy (Cryo-EM) – Captures exosomes in their native hydrated state.
- Nanoparticle Tracking Analysis (NTA) – Determines size distribution and concentration.
- Dynamic Light Scattering (DLS) – Measures hydrodynamic diameter and polydispersity index.
- Lysis-Based Purity Assessment – Quantifies lipid bilayer-enclosed exosomes by comparing nanoparticle concentration changes before and after detergent-mediated membrane disruption.
- Fluorescent Dye-Based Purity Assay – Identifies exosomes with intact membrane structures using lipophilic dyes that selectively integrate into lipid bilayers. Subsequent single-particle analysis via nanoflow cytometry enables the differentiation of exosomes from non-membranous impurities. This approach is currently validated only for animal cell-derived exosomes.
- Western Blot & Flow Cytometry – Confirms presence of exosomal markers (CD9, CD63, CD81) and absence of cellular debris markers (GM130, Calnexin).
- Size-Exclusion Chromatography (SEC) – Physically separates exosomes from free proteins and non-vesicular components.
- Sterility Testing – Detects microbial contaminants through culture-based or PCR-based assays.
- Mycoplasma Detection (qPCR & Fluorescent Staining) – Identifies mycoplasma contamination in exosome samples.
Exosomes play a vital role in intercellular communication, biomarker discovery, and therapeutic applications. However, their small size, heterogeneity, and sensitivity to environmental conditions pose challenges in research, clinical, and industrial applications. Exosome quality control (QC) is essential to ensure batch-to-batch consistency, purity, stability, and bioactivity for reproducible and effective downstream applications. MtoZ Biolabs provides a comprehensive Exosome Quality Control Service integrating cutting-edge technologies for precise exosome characterization, purity assessment, and functional validation, supporting both academic and biopharmaceutical research.
Key Exosome Quality Control Parameters and Analytical Techniques
1. Morphology & Size Distribution
Objective: Verify exosome integrity, shape, and size uniformity.
Methods:
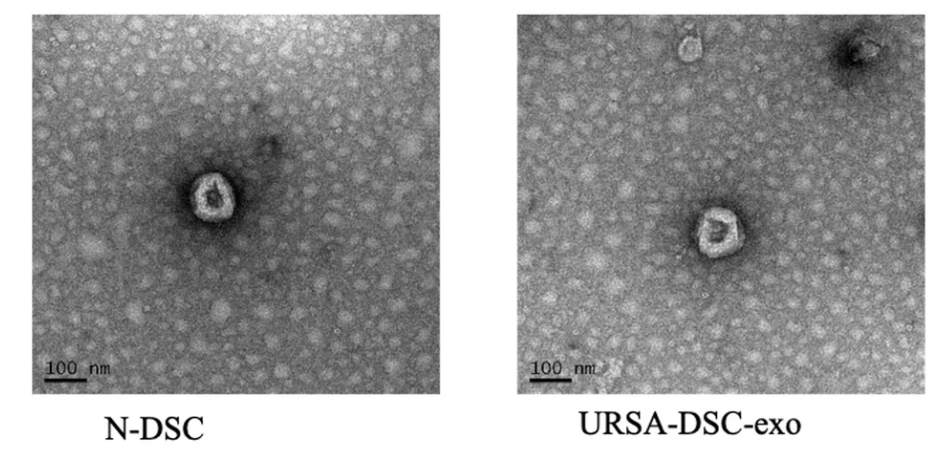
Figure 1. The Exosomes were Examined by Transmission Electron Microscopy (TEM)
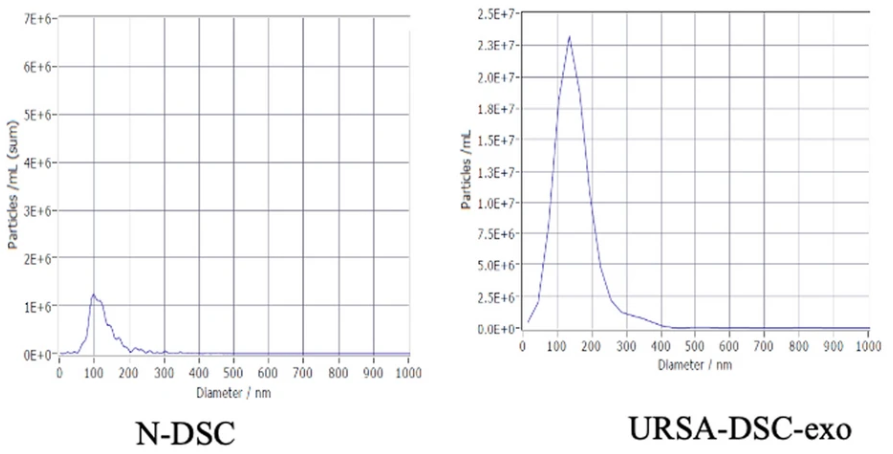
Figure 2. The Particle Sizes and Concentrations were Confirmed to be Exosomes using NTA
2. Purity & Contamination Analysis
Objective: Assess exosome purity and identify non-exosomal contaminants.
Methods:
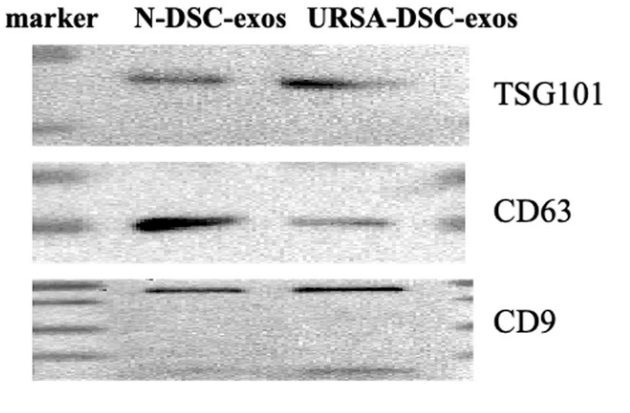
Figure 3. Western Blot Results Showed that the Expression of Marker Proteins TSG101, CD9, and CD63 was Detected in Exosomes
3. Zeta Potential Measurement
Objective: Evaluate exosome stability and colloidal dispersion behavior.
Methods: Nanoparticle Tracking Analysis (NTA) – Measures zeta potential, providing insights into surface charge and exosome stability under various buffer conditions.
4. Osmolarity Testing
Objective: Ensure exosome stability by detecting osmotic stress.
Methods: Osmometer Analysis – Evaluates buffer osmolarity to prevent exosome aggregation or degradation.
5. Microbial & Mycoplasma Contamination Testing
Objective: Ensure sterility of exosome preparations for clinical and research use.
Methods:
6. Endotoxin Contamination Testing
Objective: Quantify endotoxin levels to ensure safety in therapeutic applications.
Methods: Limulus Amebocyte Lysate (LAL) Assay – Detects exosome endotoxins with high sensitivity.
7. Heavy Metal Contamination Analysis
Objective: Ensure exosomes are free from toxic heavy metal residues that could impact biological function.
Methods: Atomic Absorption Spectroscopy (AAS) – Accurately detects trace levels of heavy metals, including lead, cadmium, arsenic, and mercury, ensuring exosome safety for downstream applications.
Service Advantages
✔️Comprehensive Quality Assessment: Utilizing advanced techniques for morphology, purity, stability, and contamination detection to ensure exosome integrity and functionality.
✔️Customized Testing Solutions: Tailoring exosome quality control protocols based on specific research or therapeutic needs, ensuring exosomes meet intended application standards.
✔️Rapid and Reliable Turnaround: Providing efficient and high-throughput exosome quality control workflows to accelerate project timelines without compromising accuracy.
✔️Expert Technical Support: Led by experienced scientists in exosome biology, quality control, and omics analysis to provide guidance and result interpretation.
Applications
1. Biomedical Research
Exosome Quality Control Service ensures exosome purity, size, and biomarker consistency for experimental reproducibility.
2. Therapeutic Development
Validates exosome safety, potency, and stability for clinical-stage therapies.
3. Biomarker Discovery
Standardizes exosome isolation and profiling using Exosome Quality Control Service for reliable biomarker identification.
4. Drug Delivery Optimization
Assesses drug-loading efficiency and exosome integrity for targeted delivery systems.
Ensuring the quality and purity of exosomes is essential for their successful application in research, diagnostics, and therapeutic development.By offering tailored solutions, rapid turnaround, and expert support, MtoZ Biolabs provides reliable and reproducible Exosome Quality Control Service. Contact us to explore tailored solutions for your research.
How to order?







