Electrochemical Corrosion Analytical Service
Electrochemical corrosion analytical is a corrosion performance evaluation method based on electrochemical techniques, primarily used to investigate the corrosion behavior and mechanisms of metals or conductive materials under specific environmental conditions. This analysis is performed by constructing an electrochemical cell system (working electrode, reference electrode, and counter electrode). Under controlled potential or current conditions, electrochemical parameters such as open circuit potential, polarization curves, and electrochemical impedance spectroscopy are measured to assess corrosion rate, corrosion resistance, and the kinetic characteristics of the corrosion process.
The electrochemical corrosion analytical service is widely applied in fields such as aerospace, automotive manufacturing, civil engineering, new energy (e.g., lithium-ion batteries, fuel cells), marine engineering, biomedical materials, and anticorrosive coating development. It is particularly suitable for applications such as material screening, validation of surface treatment processes, optimization of corrosion protection systems, and simulation of service environments. This technique is a critical tool for failure analysis and product reliability research.
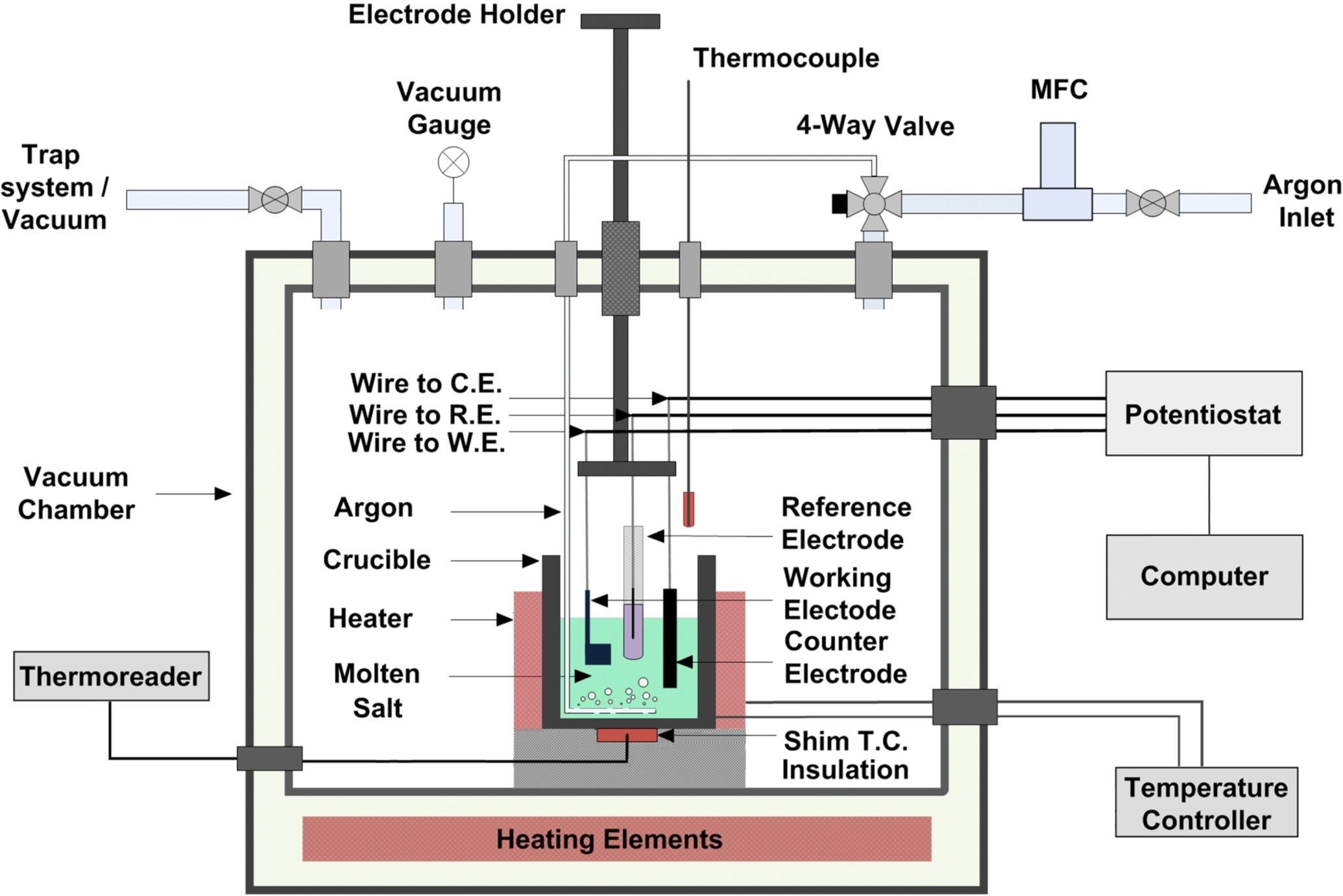
Ghaznavi, T. et al. Journal of The Electrochemical Society, 2022.
Figure 1. Schematic Diagram of the Experimental Setup for the Electrochemical Corrosion Experiments.
Services at MtoZ Biolabs
Based on a high-precision electrochemical workstation integrated with a three-electrode system, MtoZ Biolabs offers electrochemical corrosion analytical service that enable quantitative analysis of material corrosion behavior under controlled environmental conditions. The service includes open-circuit potential (OCP) measurement, electrochemical polarization curve (Tafel) testing, and electrochemical impedance spectroscopy (EIS), ensuring accurate acquisition of key parameters such as corrosion current density, corrosion potential, and charge transfer resistance. This allows for comprehensive evaluation of material corrosion resistance and corrosion mechanisms. It is widely applicable to scientific research, material screening, and anticorrosion process optimization. In addition to conventional corrosion analysis, the service also extends to the following electrochemical testing applications:
1. Photoelectrochemical Performance Testing
Used to evaluate the electrochemical response of photoactive materials (such as photocatalysts and solar cells) under different light conditions. Techniques such as linear sweep voltammetry (LSV) and transient photocurrent measurements are employed to assess charge separation efficiency and carrier dynamics.
2. Fuel Cell Performance Testing
Involves the use of polarization curves and electrochemical impedance spectroscopy (EIS) to evaluate the electrocatalytic activity, conductivity, and reaction stability of fuel cell anode or cathode materials, particularly in hydrogen or methanol fuel systems.
3. Electrocatalytic Testing
Designed to investigate materials’ catalytic efficiency and stability in key electrochemical reactions such as the oxygen reduction reaction (ORR), hydrogen evolution reaction (HER), and oxygen evolution reaction (OER). Commonly used techniques include cyclic voltammetry (CV) and chronoamperometry (CA).
Analysis Workflow
1. Sample Preparation
The test material is machined into standard-sized electrode specimens, then cleaned and polished before being mounted in the electrochemical test holder to ensure a clean, stable, and reproducible surface.
2. Electrolyte Preparation
An appropriate simulated corrosive environment (e.g., NaCl or H₂SO₄ solution) is selected based on the test requirements. Electrolyte parameters such as temperature, pH, and ionic strength are carefully controlled.
3. System Assembly and Calibration
A three-electrode system is employed, consisting of a working electrode (sample), a reference electrode (e.g., Ag/AgCl), and a counter electrode (e.g., platinum wire). The electrochemical workstation is used for wiring and parameter calibration.
4. Electrochemical Testing
Depending on the analysis goal, appropriate test methods are selected, including open-circuit potential (OCP) measurement, polarization curves (Tafel plots), electrochemical impedance spectroscopy (EIS), and cyclic voltammetry (CV), to acquire current-voltage or impedance data.
5. Data Processing and Report Generation
Collected data are fitted and analyzed to extract corrosion potential, corrosion current density, impedance characteristics, and other parameters. A comprehensive test report is generated to support corrosion behavior evaluation and comparative analysis of materials.
Advantages and Limitations
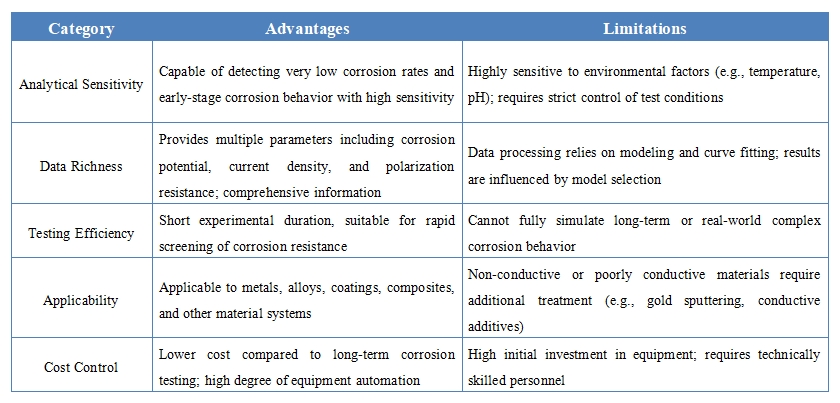
Applications
1. Corrosion Resistance Evaluation of Metallic Materials
Electrochemical corrosion analytical service can be used to assess the corrosion behavior of steels, aluminum alloys, copper alloys, and other metals in various environments (such as seawater or acidic/alkaline solutions), providing guidance for material selection and protection strategies.
2. Verification of Anticorrosion Coatings and Surface Treatments
By using polarization curves and electrochemical impedance spectroscopy (EIS), this service evaluates the barrier properties, compactness, and long-term protective performance of coatings, helping optimize formulation and processing techniques.
3. Stability Analysis of New Energy Materials
Electrochemical corrosion analytical service is applicable to materials used in lithium-ion batteries, fuel cell catalysts, and similar systems, assessing corrosion behavior and interfacial stability to ensure device performance and lifespan.
4. Simulated Environmental Corrosion Studies
Combined with environmental simulation systems such as salt spray, high humidity, or acid rain exposure, this service enables accelerated evaluation of electrochemical corrosion responses under service-like conditions.
5. Failure Analysis and Corrosion Mechanism Studies
The electrochemical corrosion analytical service supports scientific or industrial failure investigations by identifying corrosion types—such as pitting, crevice corrosion, or intergranular corrosion—and clarifying the associated reaction mechanisms.
Case Study
1. Magnetic Field Effects on the Corrosion and Electrochemical Corrosion of Fe83Ga17 Alloy
This study aimed to evaluate the influence of an external magnetic field on the electrochemical corrosion behavior of Fe₈₃Ga₁₇ alloy, a material known for its magnetostrictive properties. The research focused on Fe₈₃Ga₁₇ alloy samples and employed electrochemical testing methods such as potentiodynamic polarization curves and electrochemical impedance spectroscopy (EIS) to systematically analyze changes in corrosion current density, electrode kinetics, and interfacial impedance in 3.5% NaCl solution under varying magnetic field strengths. The results showed that increasing magnetic field intensity led to a rise in corrosion current density and a decrease in both polarization resistance and charge transfer resistance, indicating an acceleration of the corrosion process and a reduction in corrosion resistance. The study concluded that external magnetic fields significantly accelerate the electrochemical corrosion of Fe₈₃Ga₁₇ alloy, highlighting concerns regarding its safety in magnetically active environments.
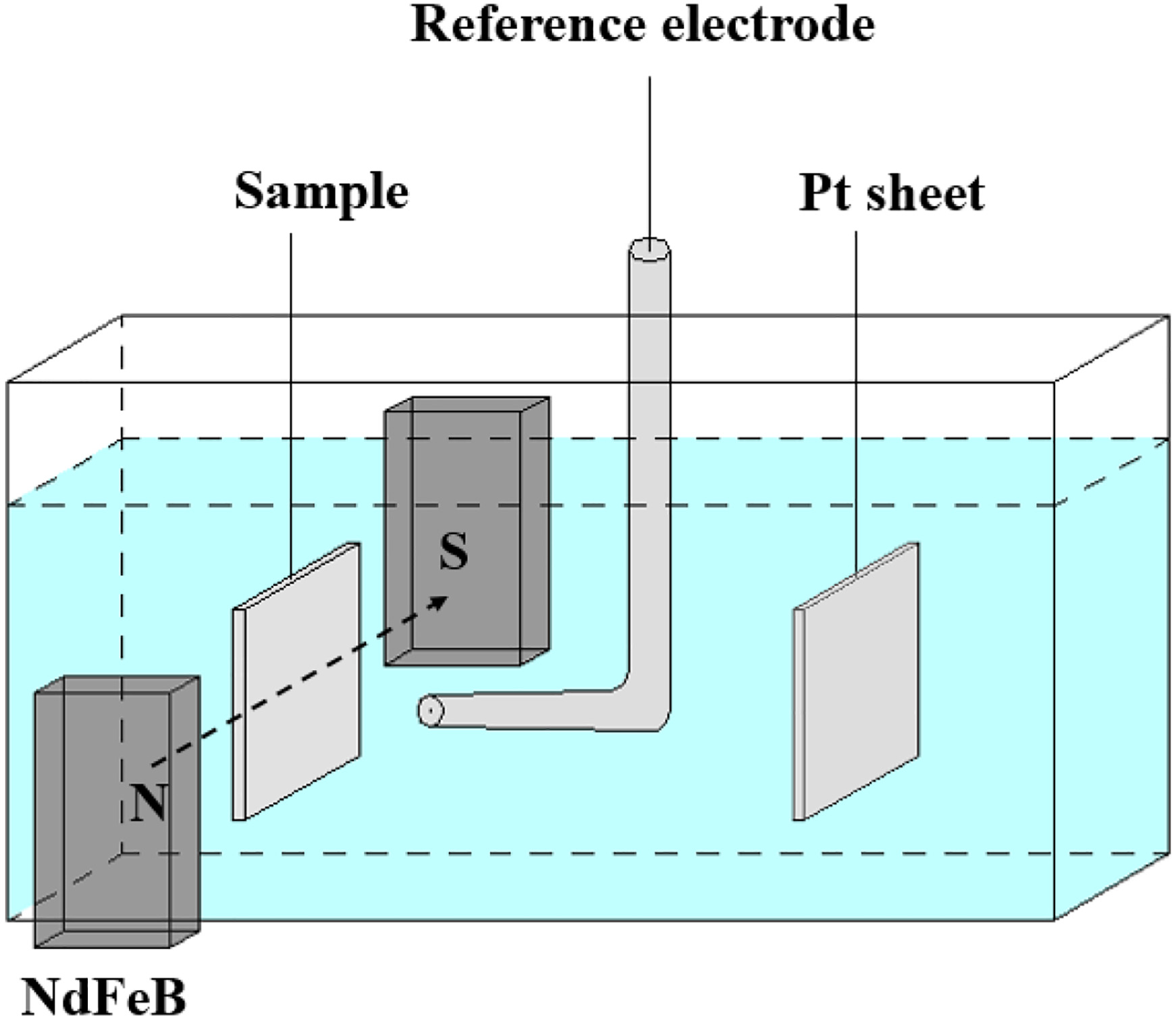
Zhao, S L. et al. Materials Characterization, 2021.
Figure 2. Schematic Diagram of the Potentiodynamic Polarization Tests.
FAQ
Q1: Are there Any Requirements for the Sample Size and Shape During Testing?
A1: It is generally recommended that the sample be flat and at least 1×1 cm² in size. If the sample is smaller or has a special structure (such as powder or coating), please contact us in advance to customize the testing plan.
Q2: Do You Support Customized Testing Protocols?
A2: Yes. We offer customized testing schemes based on the material type, corrosion medium, and application scenario, including the selection of electrochemical testing methods, temperature control, and atmospheric conditions.
How to order?







