Differences and Applications of Far-UV and Near-UV CD Spectroscopy in Protein Structure Research
-
Investigation of protein folding and unfolding
-
Stability assessments (e.g., determination of thermal melting temperature, Tm)
-
Analysis of conformational effects induced by buffer composition, pH, or ionic strength
-
Evaluation of tertiary structure integrity
-
Monitoring conformational responses before and after ligand binding
-
Comparative assessment of structural consistency across different expression systems or purification processes
-
Rapid monitoring of conformational responses during protein–small molecule interaction screening
-
Preliminary verification of conformational alterations in protein engineering mutants
-
High-throughput assessment of conformational changes in multi-component systems
Circular Dichroism (CD) spectroscopy is a sensitive and non-destructive analytical technique widely employed in protein structure research. Depending on the measurement wavelength, CD spectra are categorized into far-ultraviolet (Far-UV, 190–250 nm) and near-ultraviolet (Near-UV, 250–320 nm) regions, each offering distinct advantages for probing different levels of protein structure. This paper systematically outlines the primary differences between these two CD techniques and highlights representative applications in protein structure research.
Far-UV CD Spectroscopy: Probing Secondary Structure Features
Far-UV CD spectra predominantly arise from the differential absorption of peptide bonds to circularly polarized light, enabling accurate assessment of secondary structural elements such as α-helices, β-sheets, and random coils. Each type of secondary structure exhibits characteristic absorption peaks within the far-UV region, allowing researchers to infer the overall conformational state and monitor its structural changes.
Typical applications of Far-UV CD spectroscopy include:
Because water and conventional buffers exhibit strong absorbance between 190 and 200 nm, experiments typically employ low protein concentrations, short-pathlength cuvettes, and optimized buffer systems to ensure high-quality spectra.
Near-UV CD Spectroscopy: Assessing the Tertiary Structural Environment
Near-UV CD spectroscopy signals originate primarily from the asymmetric environments of aromatic residues (phenylalanine, tyrosine, tryptophan) and disulfide bonds, making it a valuable probe for evaluating the order and integrity of tertiary structures. Changes in this region are often indicative of local folding events, conformational rearrangements, or ligand binding.
Typical applications of Near-UV CD spectroscopy include:
Given the inherently weaker near-UV CD signals, higher sample concentrations (generally ≥1 mg/mL) and longer-pathlength cuvettes (e.g., 1 cm) are often required to achieve adequate signal-to-noise ratios.
Complementarity and Synergistic Value
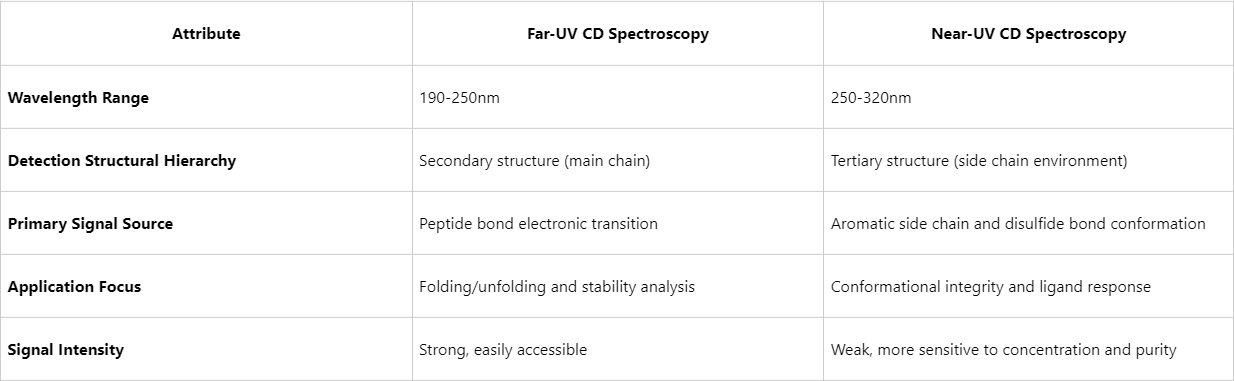
Figure1. Far-UV CD Spectroscopy vs Near-UV CD Spectroscopy
Far-UV and near-UV CD spectroscopy are complementary rather than interchangeable techniques. When applied together, they enable a comprehensive analysis of protein conformational behavior under varying conditions, from global folding states to localized structural features, thereby generating a complete map of dynamic structural changes.
The Role of CD Spectroscopy in Contemporary Protein Research
With advances in structural biology, CD spectroscopy has evolved from a routine structural screening tool into an integral component of more complex research frameworks, including:
Furthermore, the integration of computational modeling, spectral deconvolution, and machine learning algorithms is driving CD spectroscopy data toward quantitative structural interpretation and predictive capabilities, enhancing its role in high-throughput screening and quality control pipelines.
Far-UV and near-UV CD spectroscopy remain indispensable, rapid, and sensitive tools for probing protein structure research in both fundamental research and applied development. Through carefully designed experimental strategies and integrated multi-wavelength data analysis, researchers can efficiently gain structural insights without the necessity of high-resolution methods. MtoZ Biolabs combines CD spectroscopy with mass spectrometry, thermal stability assays, and other complementary techniques to deliver comprehensive protein conformation analysis solutions. We are committed to providing high-quality technical support at every critical stage of protein science research.
MtoZ Biolabs, an integrated chromatography and mass spectrometry (MS) services provider.
Related Services
How to order?







